Credit: UCL, ILL, HZB
An international team led by researchers at UCL has revealed new insights into the workings of a lithium battery by virtually “unrolling” its coil of electrode layers using an algorithm designed for papyrus scrolls.
In a study published in Nature Communications, https:/
Researchers found that using the two complementary imaging techniques and “unrolling” the electrodes while they are in normal use provides a fuller and more accurate understanding of how the battery works and how, where and why it degrades over time. Unseen trends in the spatial distribution of performance in the cells were observed.
The method paves the way for developing strategies for improving the design of cylindrical cells using a range of battery chemistries, including by informing better mathematical models of battery performance. As such the method may facilitate improvements in the range and lifetime of electric vehicles of the future.
The project was funded by the Faraday Institution, as part of its battery degradation project.
Further details
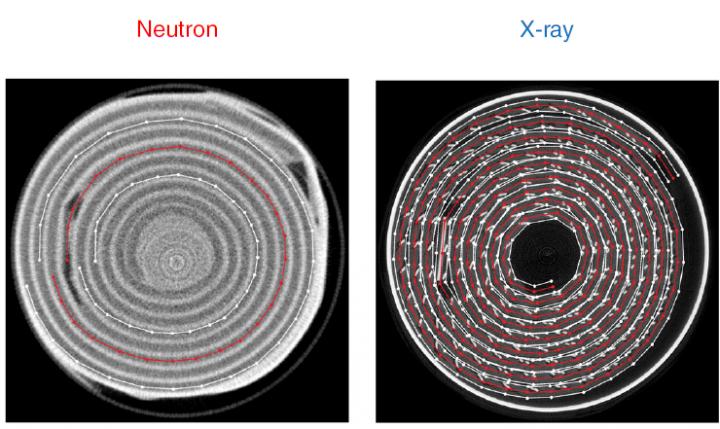
The team investigated the processes occurring during discharge of a cylindrical commercial Li-ion primary cell from Duracell using a combination of two highly complementary tomography methods. Tomography is a technique for displaying a representation of a cross section through a solid object through the use of a penetrating wave such as ultrasound or X-rays. The method is used in radiology, archaeology, atmospheric science, geophysics, oceanography as well as materials science.
X-rays are sensitive to heavier elements in the battery – such as manganese and nickel, and neutrons are sensitive to lighter elements – lithium and hydrogen, allowing the two techniques to visualise different parts of the battery structure and allowing researchers to build up a more complete understanding of the processes occurring deep within the cell during battery discharge.
X-ray computed tomography allowed for the quantification of mechanical degradation effects such as electrode cracking from the electrode bending process during cell manufacturing. Whereas the imaging using neutrons yielded information about the electrochemistry such as lithium-ion transport and consumption or gas formation by electrolyte decay.
A new mathematical method developed at the Zuse-Institut in Berlin then enabled researchers to virtually unwind the battery electrodes that are wound into the form of a compact cylinder. The cylindrical windings of the battery are difficult to examine quantitatively, and the cell cannot be unwound without inducing further damage that would not be present in an unwound battery.
###
Notes to Editors
Powering Britain’s battery revolution, the Faraday Institution is the UK’s independent institute for electrochemical energy storage science and technology, supporting research, training, and analysis. Bringing together expertise from universities and industry, the Faraday Institution endeavours to make the UK the go-to place for the research and development of the manufacture and production of new electrical storage technologies for both the automotive and wider relevant sectors.
The first phase of the Faraday Institution is funded by the Engineering and Physical Sciences Research Council (EPSRC) as part of UK Research and Innovation through the government’s Industrial Strategy Challenge Fund (ISCF). Headquartered at the Harwell Science and Innovation Campus, the Faraday Institution is a registered charity with an independent board of trustees.
The Paper and Authors
Ralf F. Ziesche1,2, Tobias Arlt3, Donal P. Finegan4, Thomas M.M. Heenan1,5, Alessandro Tengattini6,7, Daniel Baum8, Nikolay Kardjilov9, Henning Markötter3,9, Ingo Manke9, Winfried Kockelmann2, Dan J.L. Brett1,5 & Paul R. Shearing1,5*. 4D imaging of lithium-batteries using correlative neutron and X-ray tomography with a virtual unrolling technique. Nat Commun 11, 777 (2020). https:/
1 Electrochemical Innovation Lab, Department of Chemical Engineering, University College London, London WC1E 7JE, UK. 2 STFC, Rutherford Appleton Laboratory, ISIS Facility, Harwell OX11 0QX, UK. 3 Technische Universität Berlin, Strasse des 17. Juni 135, 10624 Berlin, Germany. 4 National Renewable Energy Laboratory, 15013 Denver West Parkway, Golden, CO 80401, USA. 5 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, UK. 6 Grenoble INP, CNRS, 3SR, Univ. Grenoble Alpes, 38000 Grenoble, France. 7 Institut Laue-Langevin (ILL), 71 Avenue des Martyrs, 38000 Grenoble, France. 8 Zuse Institute Berlin, Takustraße 7, 14195 Berlin, Germany. 9 Helmholtz-Zentrum Berlin für Materialien und Energie (HZB), Hahn- Meitner-Platz 1, 14109 Berlin, Germany. *email: [email protected]
Media Contact
Matt Howard
[email protected]
01-235-425-126
Original Source
https:/
Related Journal Article
http://dx.




