Credit: Tsuyoshi Kawai
Researchers at Nara Institute of Science and Technology (NAIST) report a photo-acid generator (PAG) that generates Lewis acids with a quantum yield that is vastly superior to PAGs that generate Brønsted acids. The new PAG is based on photo-chemical 6π-percyclization and is demonstrated to initiate the polymerization of epoxy monomers and catalyze Mukaiyama-aldol reactions.
PAGs are chemical species that release strong acids, either in solution or solid state, upon exposure to light. These acids can then be used to activate various biological and photo-polymer systems.
Most PAGs form Brønsted acids and do so with great efficiency. However, Brønsted acids limit options in terms of substrates and reaction mechanisms, particularly for organic synthesis when compared to Lewis acids. Some of chemical substances are easily decomposed with Brønsted acids but not with Lewis acids.
“Lewis acid catalysts are much useful but often unstable and require careful introduction to reaction systems. Preferably, we would induce Lewis acid catalysts remotely, like optical exposure,” explains NAIST Associate Professor Takuya Nakashima, one of the lead researchers in the project.
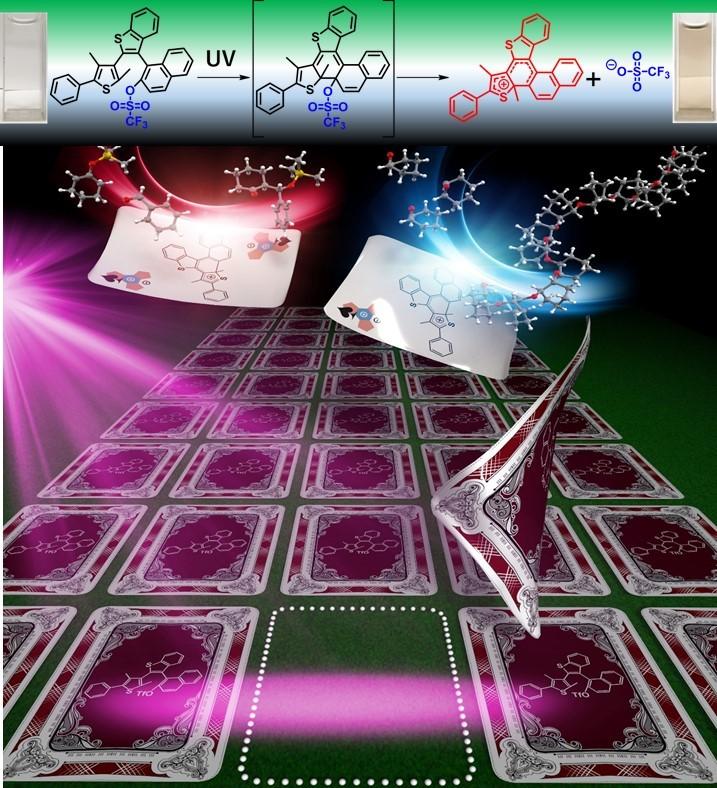
The new PAG depends on the addition of a triflate group to terarylene. The triflate group showed a high propensity to release from the terarylene upon exposure to UV light.
“We have focused on terarylene because its very high light-sensitivity, compatibility into polymer films and no oxygen/moisture inhibition. We have also found non-linear responses that can be used to greatly enhance the light-sensitivity,” says NAIST Professor Tsuyoshi Kawai, another lead researcher. Indeed, the photo-chemical quantum yield was 0.5, which is much higher than standard PAGs.
The resulting Lewis acid was generated without any radical intermediate and sustained for more than 100 days. It was then used to initiate the polymerization of epoxy monomers, SU-8, and Mukaiyama-aldol reactions for benzaldehyde and silyl enolates to produce silyl aldols of different stereoisomers, that were not possible with Brønsted acids formed with PAGs.
“This system opens new opportunities for Lewis-acid reactions. It is the first to generate photo-activated Mukaiyama-aldol reactions,” says Kawai.
###
Resource
Title: Photo-Lewis acid generator based on radical-free 6π photo-cyclization reaction
Authors: Ryo Mizutsu, Ryosuke Asato, Colin J. Martin, Mihoko Yamada, Yoshiko Nishikawa, Shohei Katao, Miku Yamada, Takuya Nakashima & Tsuyoshi Kawai
Journal: Journal of the American Chemical Society
DOI: 10.1021/jacs.9b11821
Information about Prof. Kawai lab can be found at the following website:
https:/
Media Contact
Takahito Shikano
[email protected]
Related Journal Article
http://dx.




