Utilization in material design for developing phosphor materials for white LEDs
Credit: COPYRIGHT (C) TOYOHASHI UNIVERSITY OF TECHNOLOGY. ALL RIGHTS RESERVED.
Overview
A research team consisting of Toyohashi University of Technology, Nagoya Institute of Technology, and the National Institute for Materials Science (NIMS) have clarified the mechanism by which the crystal structure of a red phosphor material obtained by adding P2O5 and Eu2O3 to a silicate (Ca2SiO4)-based material at various heat treatment temperatures changes in photoluminescence intensity due to differences in these factors.
Phosphor materials are widely used in everyday items such as vehicles and projectors in addition to LED lighting, and many researchers are developing phosphor materials using various approaches in search of brighter and more efficient phosphor materials. Knowledge of the photoluminescence intensity and crystal structure is important for designing brighter phosphor materials. In this study, the researchers succeeded in analyzing the changes in the crystal structure of the material at an atomic level due to heat treatment and the addition of P5+ and Eu3+ ions, and were able to clarify the relationships between these factors and the photoluminescence intensity.
Details
White LEDs have undergone significant development over the past two decades, the market size has continued to expand, and LEDs are now the industry leader. Phosphor materials are used in a wide range of applications such as backlighting for monitors and automotive applications in addition to lighting. In the future, many researchers will be competing to develop new materials in order to further diversify applications and realize energy saving, low cost, high color rendering, and long life, and this area has attracted a great deal of attention at academic conferences. Therefore, clarification of the characteristics and expression mechanism of phosphor materials is an important finding in the development of new materials. However, while most development of phosphor materials requires brighter and more efficient materials, there have not been many papers on the details of clarifying the mechanisms.
With this background, the research team synthesized a red phosphor made by adding P5+ , and Eu3+ as an activator to Ca2SiO4 (silicate) while changing the heat treatment temperature from 1200 to 1500 degrees. This is because the crystal structure of silicate can easily be changed by heat. Here, the activator is an element (ion) that emits various emission colors from blue to red when added to the crystal.
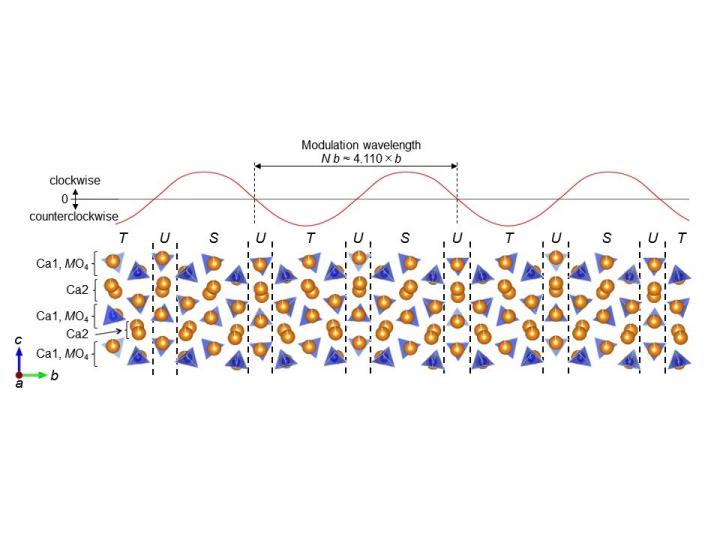
As a result of the research, it was found that the photoluminescence intensity changes depending on the heat treatment temperature, and is closely related to changes in the crystal structure. The research teams noticed that the crystal structure of the phosphor material that had been heat treated at 1500 degrees changed to an incommensurate structure (IC), which is rare for ceramic materials. Normal crystal structures have a period that is an integer multiple, but the IC phase has a non-integer multiple of modulation. The photoluminescence intensity decreased due to the formation of the IC phase. By making full use of X-ray diffraction and computational science, the teams succeeded in analyzing the crystal structure in detail at the atomic level. As a result of the analysis, the material has a modulation structure of 4.110 times in the b-direction, the structure has three types of SiO4 tetrahedron (T, U, S) gradients, and two more types of gradient (T”, S”) when looking at the long period. In this way, the teams discovered that the material constitutes an IC phase. It is thought that the reason for such a complex IC phase is that P is present in a part of the SiO4 tetrahedron, Eu is present in a part of the Ca, and the crystal structure is formed by rapid cooling treatment from a high temperature of 1500 degrees. Based on the precise analysis of the crystal structure, the researchers can answer questions such as: Which crystal sites should have an activator in order to synthesize a material with a brighter photoluminescence intensity? Which crystal structure is better? The teams believe that this knowledge can be used for new material design.
Development background
Prof. Hiromi Nakano and her team started research on phosphor materials using a silicate matrix doped with P2O5 about five years ago. Since the crystal structure of silicate can be controlled by the heat treatment temperature, she focused on the relationships between the crystal structure and the photoluminescence characteristics from the beginning of the research. In this research, when Prof. Hiromi Nakano, the team leader, showed the data to Professor Fukuda of the Nagoya Institute of Technology because the obtained XRD did not match the previous results, he advised that there was the possibility of an IC phase, and that the IC phase could also be analyzed by XRD. Prof. Nakano observed through electron diffraction that there was a crystal structure with a non-integer multiple period, but other means were needed to obtain quantitative data. The research collaboration between the group of XRD experts led by Professor Fukuda at Nagoya Institute of Technology and the IC phase analysis group led by Japan’s leading analyst for IC phase, Dr. Michiue at NIMS resulted in the successful precise analysis of the crystal structure.
Future outlook
This knowledge is important for the development of phosphor materials, and the researchers believe it will be useful in industry in this field. Going forward, the researchers intend to conduct precise crystal structure analyses, further develop new materials, and widely disclose the new knowledge related to the physical properties of phosphor materials.
###
Reference
Hiromi Nakano, Shota Ando, Konatsu Kamimoto, Yuya Hiramatsu, Yuichi Michiue,
Naoto Hirosaki and Koichiro Fukuda, “Incommensurately Modulated Crystal Structure and
Photoluminescence Properties of Eu2O3- and P2O5-Doped Ca2SiO4 Phosphor”, (2020) Materials, 13, 58; doi:10.3390/ma13010058.
Media Contact
Yuko Ito
[email protected]
Related Journal Article
http://dx.




