Credit: Ames Laboratory, US Department of Energy
Scientists at the U.S. Department of Energy's Ames Laboratory are being credited with creating the first intermetallic double salt with platinum.
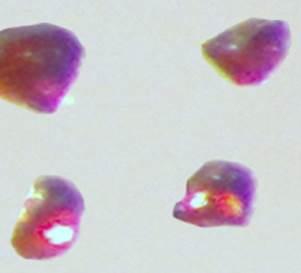
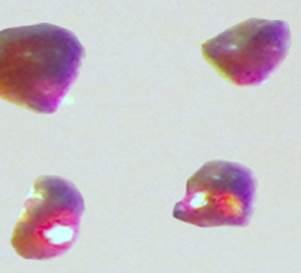
Materials researchers Anja-Verena Mudring and Volodymyr Smetana were the first to create and accurately characterize the compound. Cesium platinide hydride, or 4Cs2Pt?CsH, forms a translucent ruby red crystal and can exist only in an inert environment similar to conditions that exist in outer space. It's a new member of a rare family of compounds in which a metal forms a truly negatively charged ion.
"It's a compound that as a researcher you have trouble envisioning that it can even exist, but once you do have it and can analyze it, it's nothing like what you expect," said Mudring. "Instead of creating a gray, shiny alloy as typically observed for many hydrogen storage materials by reacting the metals cesium and platinum with hydrogen, these red crystals form. They are really quite beautiful."
This intriguing new compound was initially extracted from a cesium melt. The compound is highly unstable, with the platinum in the compound returning to its elemental state if it is exposed to oxygen. Since first detected it took a long time to understand its true nature and prove the composition. Single crystal studies combined with powder X-ray diffraction, solid-state nuclear magnetic resonance and deep theoretical investigations allowed researchers to prove its existence. Its unusual structure and properties, so different from typical intermetallic hydrides, are explained by the strong influence of relativistic effects on both cesium and platinum.
"It's unique. It's the first example we have of a salt with so strongly negatively charged metal ions. Moreover, you mix an alloy with a salt and get another non-conducting salt," said Mudring. "This allows for some deep insight into the nature of chemical bonding–and as Goethe wrote, ultimately what holds the world and its compounds together in its inmost form."
The research is further discussed in a paper published in and featured on the cover of the current issue of Angewandte Chemie, "Cesium Platinide Hydride 4Cs2Pt?CsH: An Intermetallic Double Salt Featuring Metal Anions," by Volodymyr Smetana and Anja-Verena Mudring.
###
Ames Laboratory is a U.S. Department of Energy Office of Science national laboratory operated by Iowa State University. Ames Laboratory creates innovative materials, technologies and energy solutions. We use our expertise, unique capabilities and interdisciplinary collaborations to solve global problems.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Media Contact
Laura Millsaps
[email protected]
@Ames_Laboratory
http://www.external.ameslab.gov
############
Story Source: Materials provided by Scienmag