Credit: Hyung Joon Cha
Among the Marvel characters, Spider-man has been the most popular character for the longest time of its history. The most attractive superpower of the Spider-man is that he shoots sticky spider webs to cling to walls or fly between buildings. Would his spider web be powerful in underwater, too? The answer is no. Because spider webs are dissolved in water and show no longer strong adhesion. However, mussels are capable of strong underwater adhesion. It is not even affected by heavy waves or storms. When a mussel is removed from a rock by force, surface of rock is also teared. That’s how strong its adhesion is. Mussels produce tough fibers called byssus to attach to surface of a rock and, adhesive proteins are secreted when mussels make byssus.
Hyung Joon Cha, professor of POSTECH Chemical Engineering Department and his research team of Jeong Woo Han (Prof.) and Mincheol Shin collaborated with Nak-kyoon Kim of Korea Institute of Science and Technology on studying adhesive proteins in a mussel. They analyzed adhesive proteins secreted by mussels and confirmed two molecules, Dopa and Lysine which have strong adhesion even in underwater. Also, they discovered that these molecules have synergetic effect on mussel adhesions in various conditions. Their findings took one step closer in unveiling the secret of underwater mussel adhesion.
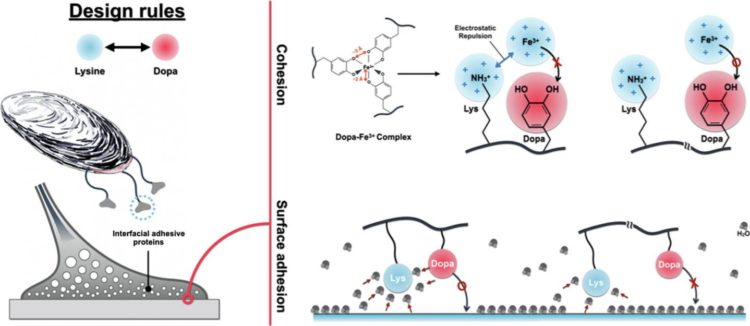
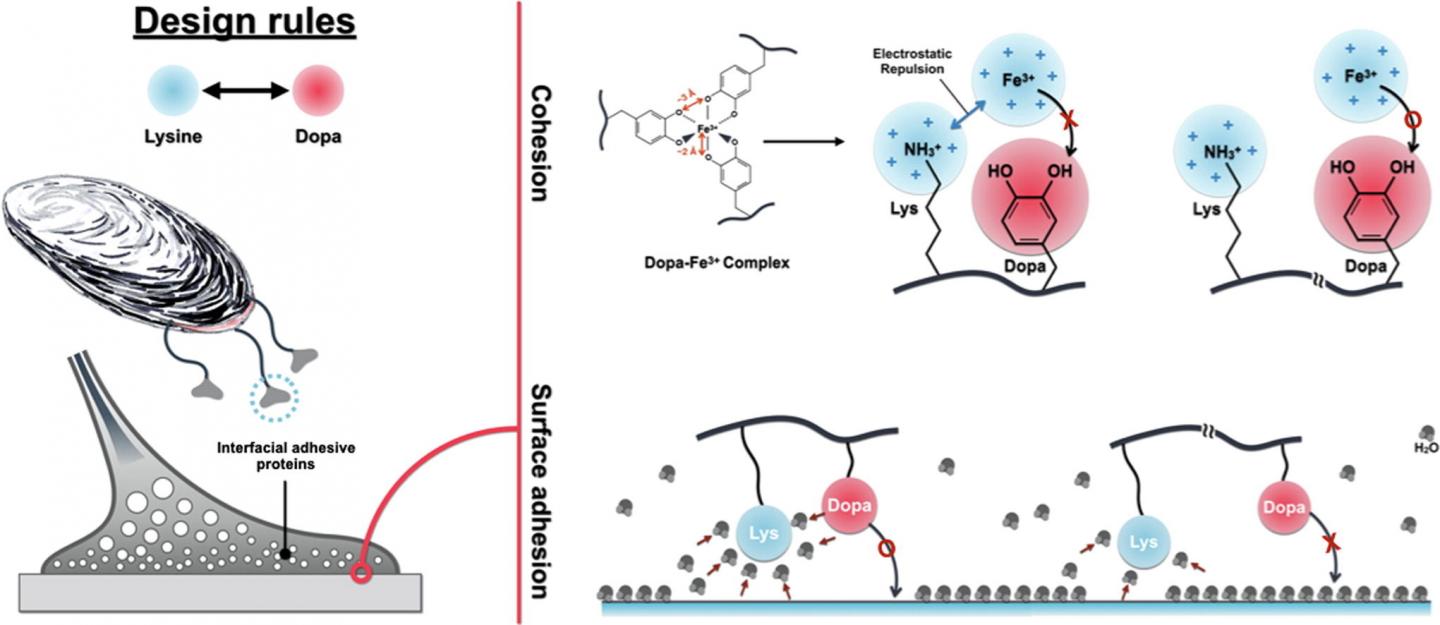
Before their findings, researchers paid attention to a molecule, Dopa. The shape of Dopa has been imitated to make underwater adhesives. But, there has been a limitation in making underwater adhesives as strong as nature adhesives of mussels because it has been challenging to balance between surface adhesion, which is attraction between surface and adhesives, and cohesion of adhesive molecules.
Unlike the conventional studies, the research group of Cha recognized an important role of another molecule called Lysine in underwater mussel adhesion. The interfacial adhesive protein, fp-3F, is located in surface of mussels and contains a great quantity of Dopa, which makes underwater adhesion possible and Lysine, which has a positive electric charge. The joint research team observed distribution of these molecules in the protein and found an interesting fact that these molecules were either bound to or apart from each other at a specific location.
Based on the sequencing of an interfacial adhesive protein, they synthesized three simple peptides with each different distance between Dopa and Lysine. By testing these model peptides, they discovered that the distance between Dopa and Lysine affected their synergy on surface adhesion and cohesion differently.
First of all, when these two molecules were adjacent to each other, surface adhesion of the peptide increased greatly. The team confirmed that Lysine enhanced underwater surface adhesion by attracting water molecules, which disrupted underwater adhesion, in the surface and water molecules around Dopa.
Next, they noticed that ferric ion(Fe3+)-mediated cohesion diminished unlike the surface adhesion when Lysine was flanked to Dopa. They explained that this was because Lysine disrupted ferric ion, a mediator for cohesion, from approaching Dopa electrically and structurally.
Through molecular biology techniques, they synthesized two different proteins with artificial sequences and compared with proteins with natural sequence to apply the same mechanism in interfacial adhesive proteins of a mussel. As a result, they verified that the identical result was obtained in the proteins with artificial sequences.
Professor Cha said, “We discovered synergy of two molecules, Dopa and Lysine which are known to play important roles in underwater adhesion. With this accomplishment, we anticipate to see new underwater bioadhesives on another level.” Also, this research explained how adhesive proteins of a mussel are designed and could enlighten future studies of other adhesive proteins in nature.
Their research accomplishment has been recently posted in Journal of Colloid and Interface Science, the world’s prominent journal in the field of interface science. The research was supported by the Basic Science Research Program through the National Research Foundation of Korea.
###
Media Contact
Jinyoung Huh
[email protected]
82-542-792-415
Original Source
http://postech.
Related Journal Article
http://dx.