Medical University of South Carolina researchers use the colloquially known ‘love hormone’ oxytocin as potential therapeutic for cocaine addicts with childhood trauma, discovering key gender differences in response
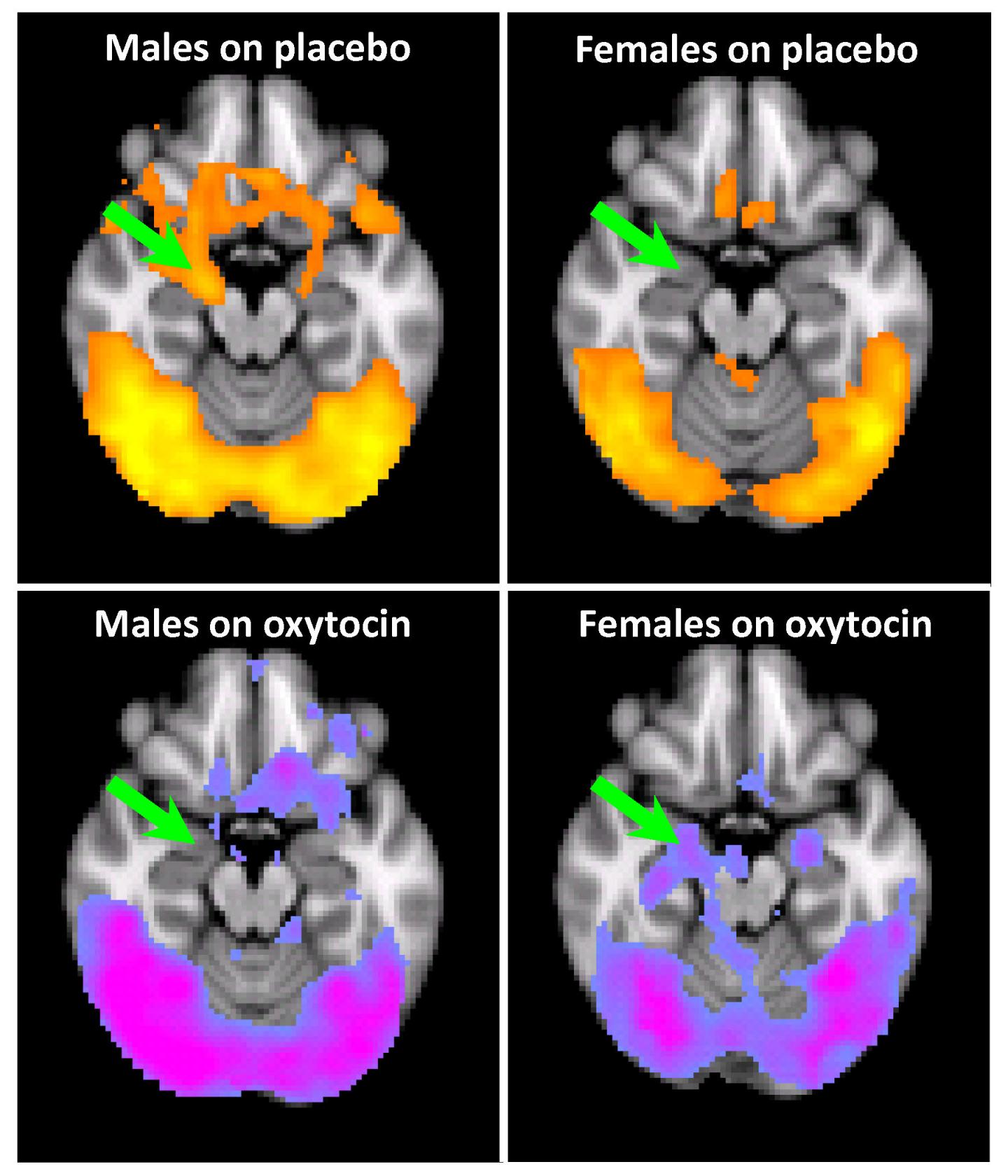
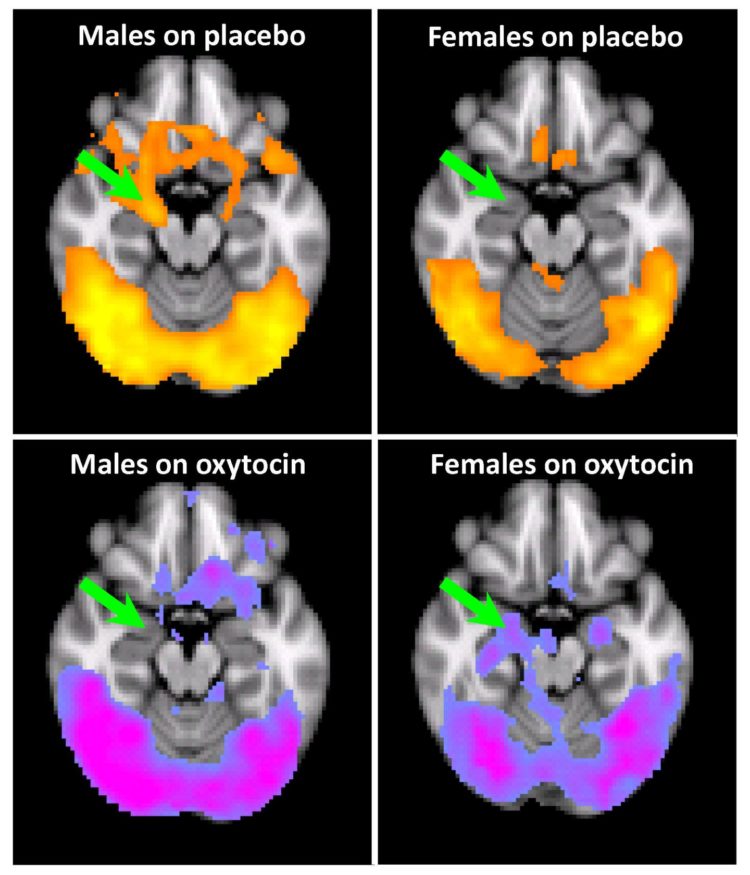
Credit: Image abridged from figure in Joseph et al Psychopharmacology article (DOI: 10.1007/s00213-019-05360-7), with permission of Springer Nature.
A team of addiction researchers at the Medical University of South Carolina (MUSC) report in Psychopharmacology that oxytocin, a hormone produced naturally in the hypothalamus, has significant gender differences when used as a treatment for cocaine-addicted individuals with a history of childhood trauma.
The MUSC team was led by Jane E. Joseph, Ph.D., a professor in the Department of Neuroscience, and Kathleen T. Brady, M.D., Ph.D., a professor in the Department of Psychiatry and Behavioral Sciences and MUSC vice president for research.
Oxytocin has been shown to have therapeutic action in addiction, reducing cravings that could result in a relapse while also reducing brain activity associated with stress. Despite previous studies showing some potential therapeutic actions of oxytocin, it was not yet known how oxytocin influenced cravings induced by the sight of cocaine paraphernalia or whether gender-based differences existed.
To understand the role of oxytocin in addiction, it is important to understand the changes that can occur in the brain in response to environmental factors. Extremely traumatic events such as childhood trauma can lead to post-traumatic stress disorder, which can change neural connections within the brain. Addiction can lead to changes in the connections of the brain as well, and the areas changed by both trauma and addiction can overlap.
The amygdala, one region in the brain that experiences these changes, is rich in oxytocin receptors and can become hyperreactive in response to stress. While oxytocin has been shown to reduce amygdala activity in response to stress cues, less was known about how oxytocin might affect cravings for cocaine in addicted individuals.
To test the craving response, the researchers asked 67 study participants, while in an MRI, to view images of drug paraphernalia alongside images of more mundane items. Viewing images of drug paraphernalia led to the amygdala “lighting up” in drug-addicted men, correlating to an increase in cravings for cocaine. Participants were then each treated with either oxytocin or a placebo, and the effect on the amygdala was measured.
In men with a history of trauma, the response was as predicted. Oxytocin reduced the activity within the amygdala, as well as the cravings that the individuals felt for cocaine, consistent with previous studies demonstrating the hormone’s therapeutic effect.
Surprisingly, this did not hold true for women with a history of trauma. While the amygdala of men with cocaine addiction would become highly active in response to visual drug cues, that of women with cocaine addiction and a history of trauma showed little activity.
“When the women with trauma were viewing the cocaine cues on placebo, they didn’t have a strong response to begin with, which was surprising,” said Joseph. “In fact, the treatment with oxytocin led to the response in the brain to drug paraphernalia being enhanced and exacerbated.”
Historically, cocaine-addicted women have worse treatment outcomes than their male counterparts. This study clearly points to the need to flesh out the trauma-induced changes in the brain, explore how they differ by gender and better understand how they affect addiction. It also suggests that treating women with a history of childhood trauma with oxytocin alone might increase both amygdala activity and cravings, potentially leading to a higher incidence of relapse.
“Even though more men use cocaine, it really has more devastating effects on women when they relapse, and they’re much more sensitive to cocaine,” said Joseph.
Joseph sees several plausible explanations for the study’s surprising findings that gender matters when it comes to the response of cocaine-addicted individuals, with a history of trauma, to oxytocin treatment. Men may be more susceptible to the visual cues of drug paraphernalia and the cravings that they induce. In contrast, women may be more susceptible to “stress-associated” cues, such as visuals that relate to past traumas, which could increase amygdala response. Alternatively, women might have a blunted response in the amygdala to stress and cravings as a result of changes that could occur in response to the initial trauma-induced hyperreactivity. However, as this study only looked at drug cues and craving responses, these hypotheses must be evaluated in future studies.
Existing therapies for addiction may not have been developed with attention to how gender affects treatment responses, perhaps accounting in part for the increased rates of treatment failures in women. Better understanding of the intricacies of trauma, addiction and gender differences could move addiction researchers one step closer to finding effective and personalized therapies for everyone.
“The search for a medication to treat cocaine use disorder has been unsuccessful to date,” said Brady. “Exploring subgroups of individuals, such as those with childhood trauma, and agents with novel mechanisms of action, like oxytocin, is critical to moving the field forward.”
###
The study was completed with support from the National Institute on Drug Abuse and the South Carolina Clinical & Translational Research (SCTR) Institute, a Clinical and Translational Science Awards hub headquartered at MUSC. SCTR provided data management and the collection and processing of patient samples through the Nexus Research Center.
About the Medical University of South Carolina
Founded in 1824 in Charleston, the Medical University of South Carolina (MUSC) is the oldest medical school in the South, as well as the state’s only integrated, academic health sciences center with a unique charge to serve the state through education, research and patient care. Each year, MUSC educates and trains more than 3,000 students and 700 residents in six colleges: Dental Medicine, Graduate Studies, Health Professions, Medicine, Nursing and Pharmacy. The state’s leader in obtaining biomedical research funds, in fiscal year 2018, MUSC set a new high, bringing in more than $276.5 million. For information on academic programs, visit http://musc.
As the clinical health system of the Medical University of South Carolina, MUSC Health is dedicated to delivering the highest quality patient care available while training generations of competent, compassionate health care providers to serve the people of South Carolina and beyond. Comprising some 1,600 beds, more than 100 outreach sites, the MUSC College of Medicine, the physicians’ practice plan and nearly 275 telehealth locations, MUSC Health owns and operates eight hospitals situated in Charleston, Chester, Florence, Lancaster and Marion counties. In 2019, for the fifth consecutive year, U.S. News & World Report named MUSC Health the No. 1 hospital in South Carolina. To learn more about clinical patient services, visit http://muschealth.
MUSC and its affiliates have collective annual budgets of $3 billion. The more than 17,000 MUSC team members include world-class faculty, physicians, specialty providers and scientists who deliver groundbreaking education, research, technology and patient care.
About the South Carolina Clinical and Translational Research Institute
The South Carolina Clinical and Translational Research (SCTR) Institute, a National Institutes of Health Clinical and Translational Science Awards hub, is the catalyst for changing the culture of biomedical research, facilitating sharing of resources and expertise, and streamlining research-related processes to bring about large-scale, change in the clinical and translational research efforts in South Carolina. Our vision is to improve health outcomes and quality of life for the population through discoveries translated into evidence-based practice. For more information, visit https:/
Media Contact
Heather Woolwine
[email protected]
843-792-7669
Original Source
https:/
Related Journal Article
http://dx.