Lithium-ion batteries have revolutionised technology and mobile devices, and their use today is standard in mobile phones and laptop computers
Credit: University of Seville
Researchers from the University of Oxford and the University of Seville have published a study in the review Nature in which they define new strategies for the manufacturing of a generation of safer and more efficient lithium-ion batteries. In this way, they intend to overcome some of the limitations that these devices currently have. Their storage capacity and the pollution caused by some of the materials used to make them.
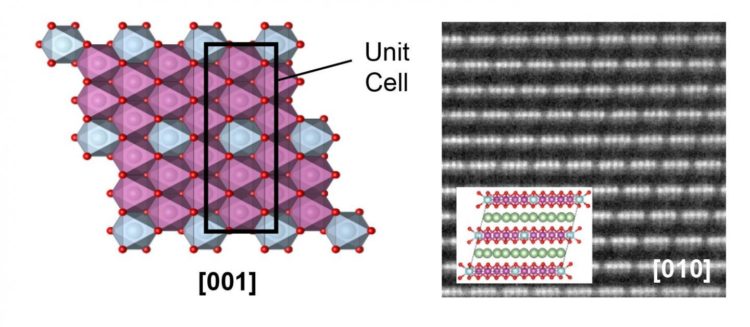
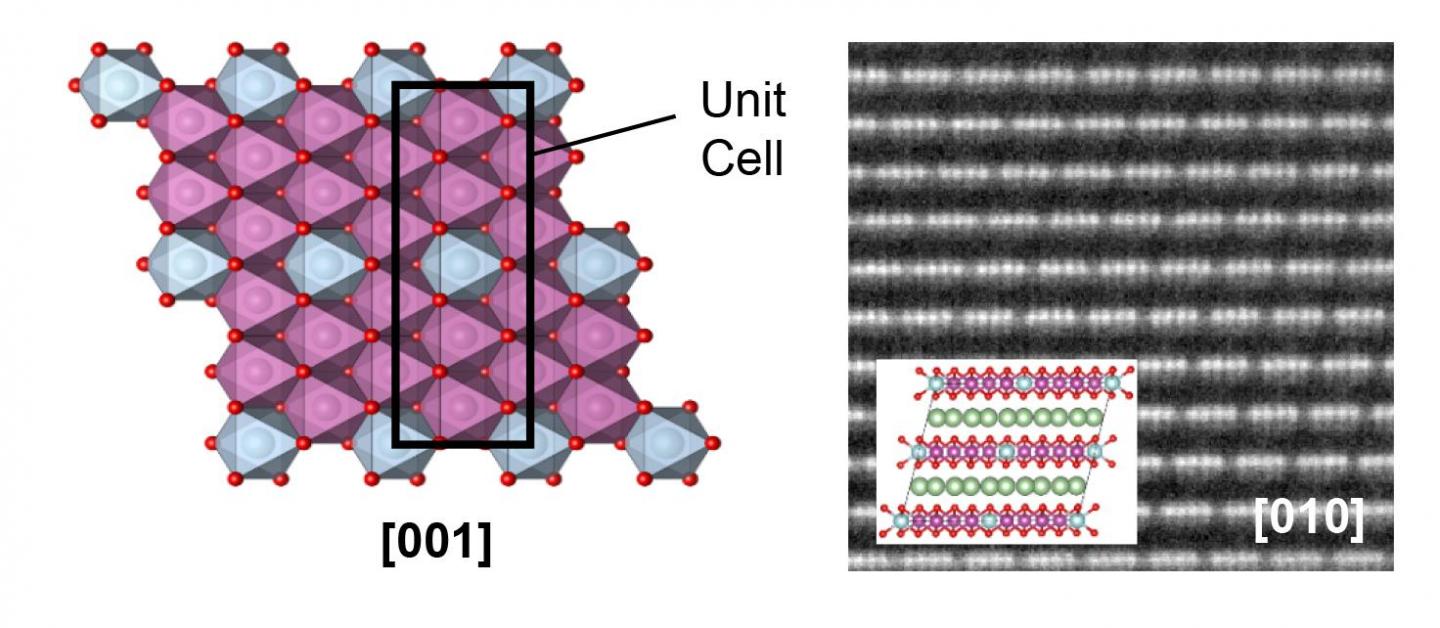
To that end, they have studied two types of cathodes that are very similar in their composition, but which show completely different behaviour: one of them suffers from the known loss of energy density in the first charge cycle, while the other does not. “This different in behaviour is owed to the formation in one type of a new superstructure, that is to say, to a very particular ordering of the metal atoms”, explains Juan Gabriel Lozano, researcher from the Higher Technical School of Engineering at the University of Seville. It has been shown that this characteristic prevents restructuring during the first charge cycle. “This result will allow us to overcome one of the principle bottlenecks that has, until now, been met in the development of this type of technology”, the researcher explains.
Lithium-ion batteries have revolutionised portable technology, and their use today is standard in mobile phones, laptop computers, etc. In recognition of that fact, the creators of this type of battery have this year received the Nobel Prize for Chemistry. However, there are still specific problems that have to be resolved. Firstly, “conventional Li-ion batteries are not capable of storing sufficient energy for their use to extend to grater uses such as in electric cars. In addition, in their cathodes, the majority use metals that are toxic, polluting and have associated safety problems. For all those reasons, there is a growing interest in the scientific community in developing new materials that solve these problems”, explains the University of Seville researcher.
Among the strongest candidates for replacing part of the current technology, are so-called lithium transition metal oxides. These cathodes are capable of storing a greater energy density than conventional ones, and are safer, cheaper and less harmful to the environment. However, they have a fundamental problem, and that is that a good part of the energy density is lost in the first charge cycle. This problem, connected to the atomic restructuring of the cathodes during the extraction and insertion of lithium, has meant that their implementation has still not been possible. However, the finding discussed in this article will allow this difficulty to be overcome.
###
Caption: Images from a high-resolution scanning electron microscope, and atomic models in which can be observed the atomic structure of the cathodes that suffer energy-density loss in the first charge cycle (left) and the new superstructure that prevents this loss (right).
Media Contact
Juan Gabriel Lozano Suarez
[email protected]
Original Source
https:/
Related Journal Article
http://dx.