Scientists piece together the inflammation mechanism in IgG4-related disease, an autoimmune condition with no current cure, revealing possible therapeutic targets
Credit: Tokyo University of Science
Autoimmune diseases are a medical conundrum. In people with these conditions, the immune system of the body, the designated defense system, starts attacking the cells or organs of its own body, mistaking the self-cells for invading disease-causing cells. Often, the cause for this spontaneous dysfunction is not clear, and hence, treatment of these diseases presents a major and ongoing challenge.
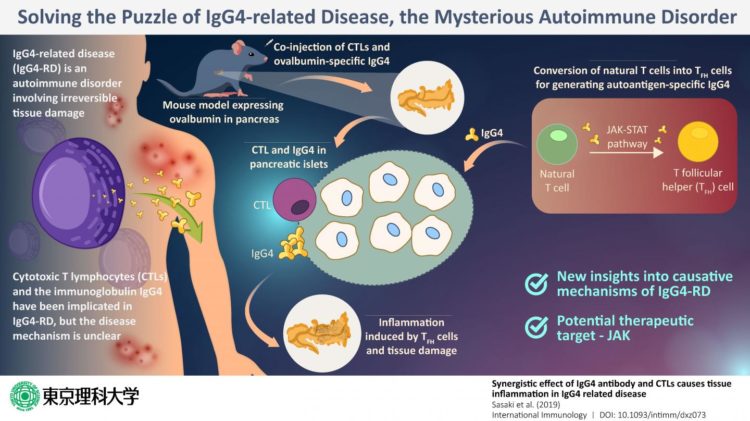
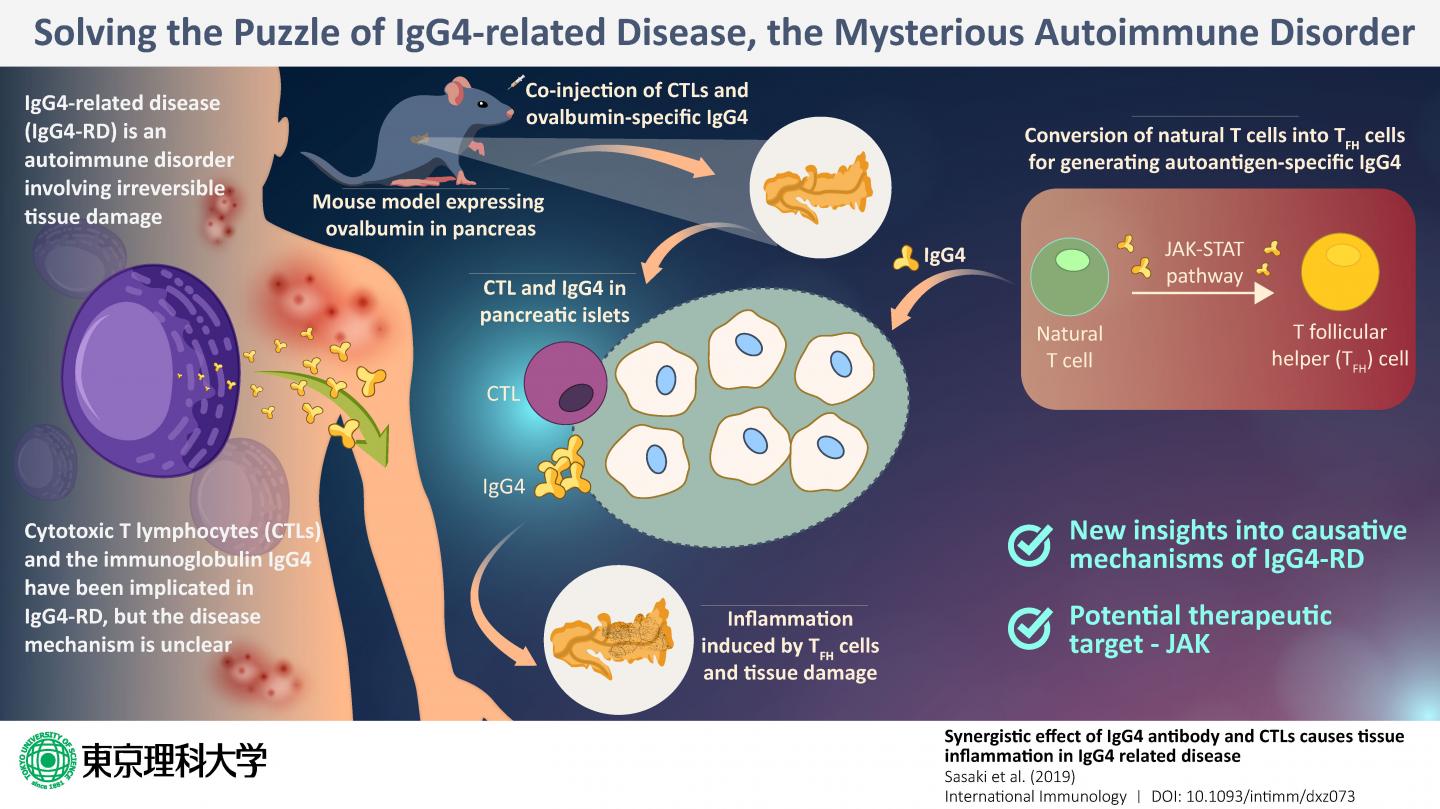
One recently discovered autoimmune disease is the IgG4-related disease (or IgG4-RD), which involves the infiltration of plasma cells that are specific to the immunoglobulin (antibody) IgG4 into the body tissue, resulting in irreversible tissue damage in multiple organs. In most patients with IgG4-RD, the blood levels of IgG4 also tend to be higher than those in healthy individuals. Previous studies show that T cells–which are white blood cells charged with duties of the immune response–play a key role in the disease mechanism. In particular, special T cells called cytotoxic T lymphocytes, or CTLs, were found in abundance from the inflamed or affected pancreas of patients, along with IgG4. But what was the exact role of CTLs?
In a new study published in International Immunology, a team of scientists from Tokyo University of Science decided to find the answer to this question. Prof. Masato Kubo, a member of this team, states that their aim was twofold. “We planned to explore how IgG4 Abs contributes to the CTL-mediated pancreas tissue damage in IgG4-RD, and also to evaluate the pathogenic function of human IgG4 Abs using the mouse model that we have established.” The latter is especially important, as IgG4 is not naturally present in mice, meaning that there is a severe lack of adequate animal models to explore this disease.
With these aims, they selected mice that have been genetically programmed to express a protein called ovalbumin (the major protein in egg white) in their pancreas. Then, they injected IgG4 that specifically targets ovalbumin into the mice. Their assumption was that IgG4 would target the pancreas and bring about IgG-4-RD-like symptoms. However, what they found was surprising. No inflammation or any other symptom typical of IgG4-RD appeared. This convinced the researchers that IgG4 alone was not the causative factor of IgG4-RD.
Next, to check if it was the CTLs that were perhaps the villain of the story, the scientists injected both IgG4 specific against ovalbumin as well as CTLs. Now, the pancreas of the mice showed tissue damage and inflammation. Thus, it was established that the presence of CTLs and IgG4 was necessary for pancreatic inflammation.
When they probed further, they found that another variation of T cells, known as T follicular helper or “TFH cells,” which develop from the natural T cells of the mice, produce self-reactive antibodies like IgG4, which induce inflammation in combination with CTLs.
Once the puzzle was pieced together, the scientists now had the opportunity to zero in on the target step for intervention; after all, if one of these steps is disrupted, the inflammation can be prevented. After much deliberation, they propose that Janus kinase, or JAK, can be a suitable target. JAK is a key component of the JAK-STAT cellular signaling pathway, and this pathway is an integral step in the conversion of natural T cells of the mice to TFH cells. If this JAK is inhibited, this conversion will not take place, meaning that even the presence of CTLs will not be able to induce inflammation.
Prof. Kubo also suggests a broader outlook, not limited to the therapeutic option explored in the study. He states, “based on our findings, the therapeutic targets for IgG4-related diseases can be the reduction of TFH cell responses and the auto-antigen specific CTL responses. These can also provide the fundamental basis for developing new therapeutic applications.”
These proposed therapeutic targets need further exploration, but once developed, they have the potential to improve the lives of millions of patients with IgG4-RD worldwide.
###
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society”, TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https:/
About Professor Masato Kubo from Tokyo University of Science
Dr Masato Kubo is a Professor at the Tokyo University of Science. A respected and senior researcher in his field, he has more than 226 publications to his credit. He is also the corresponding author of this study. His research interests include Immunology and Allergology. He is the team leader at the Laboratory for Cytokine Regulation, RIKEN Center for Integrative Medical Sciences.
Funding information
This study was supported by grants from JSPS KAKENHI (grant no. 19H03491), Japan Agency for Medical Research and Development (AMED), AMED-CREST, and Toppan Printing CO., LTD.
Media Contact
Tsutomu Shimizu
[email protected]
Related Journal Article
http://dx.