Contributing to the realization of a sustainable society by reducing emissions of air pollutants and greenhouse gases
Credit: ©Furuya Metal
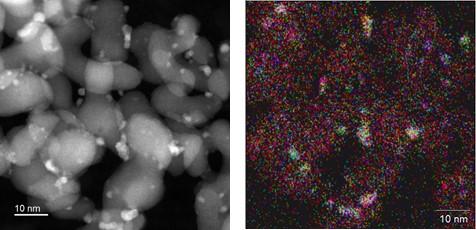
In the JST Strategic Basic Research Programs, Furuya Metal Co., Ltd. and Professor Hiroshi Kitagawa, Graduate School of Science, Kyoto University, have developed mass production technology that enables the continuous synthesis of several nm solid-solution alloy particles, which had previously been difficult to achieve. With this technology, we succeeded in achieving a stable and continuous synthesis of 1nm-class solid-solution alloy nanoparticles and their supported catalysts, which were difficult to obtain using general methods of liquid phase reduction reaction (Fig. 1).
In the conventional methods, when we try mass-producing solid-solution alloy nanoparticles, the mixing method of elements is not uniform and the particle size distribution is widened, making it difficult to synthesize continuously with good quality and stability. In order to realize mass production technology, we have newly developed a continuous-flow production system (Fig. 2) that applies the solvothermal synthesis method and introduced it to Furuya Metal Co., Ltd. This equipment enables continuous production while maintaining the quality of solid-solution alloy nanoparticles, and we are aiming for mass production based on this equipment configuration.
The newly developed solid-solution alloy nanoparticles by this synthesis device is a new alloy made of metals that had been impossible to mix together. Furthermore, it is well known in many research fields including catalytic science that the physical and chemical properties of alloys dramatically change by reducing to the nanoscale. The solid-solution alloy nanoparticles are considered as innovative catalysts that purify various exhaust gases and efficiently convert raw materials into basic chemicals and energy. Therefore, they will contribute greatly to the realization of a sustainable society in environmental purification and manufacturing technologies that emit less carbon dioxide.
In fact, it is already under evaluation process as an exhaust gas purification catalyst for automobiles and chemical process catalysts and we are promoting its implementation in the society in collaboration with domestic and foreign companies and research institutions.
Fig. 3 shows the results of a purification performance test for nitrogen oxides (NOx) contained in an automobile exhaust gas. We have succeeded in developing an inexpensive catalyst that is far superior to rhodium (Rh), which is currently used as the best catalyst, and that shows activity at low temperatures. Automotive exhaust gas purification catalysts are good at exhaust gas purification performance in the temperature range around 600°C, and there has been a great demand for improvement in exhaust gas purification performance when the engine is not immediately warmed (cold start) after it is start. The exhaust emission regulations for automobiles have been becoming stricter year by year, and even at such cold start, it is essential to improve the low-temperature activity that satisfies the regulation standards. In the evaluation of Fig. 3, the activity of Rh catalyst was also evaluated as a comparison; however, the reaction of alloy A synthesized using this technology started at a low temperature of approximately 50°C. The NOx conversion at 160°C of the solid-solution alloy nanoparticles was more than seven times higher than that of Rh, indicating it is an innovative one.
By further applying this technology, it is expected to development of new solid-solution alloy nanoparticles materials that were difficult to fabricate, and practical use of solid-solution alloy nanoparticles materials that had been without mass production technology.
###
Media Contact
Naohiro Morozumi
[email protected]
81-359-773-388
Original Source
https:/




