By successfully strengthening pancreatic islets before transplantation, researchers at UNIGE and HUG are hoping for a significant improvement in the success of cell transplants in patients with severe diabetes
Credit: © UNIGE
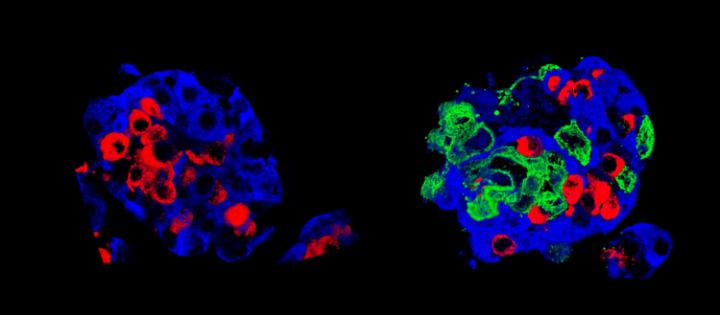
To save patients with a severe form of type 1 diabetes (characterized by the absence of functional insulin-producing cells), pancreatic cell transplantation is sometimes the last resort. The pancreas contains cell clusters – called islets of Langerhans – where cells that produce blood glucose regulating hormones are grouped together. However, the transplant process is long and complex: a significant part of the grafted cells die quickly without being able to engraft. By adding amniotic epithelial cells to these cell clusters, researchers at the University of Geneva (UNIGE) and the Geneva University Hospitals (HUG), Switzerland, have succeeded in creating much more robust “super-islets” of Langerhans. Once transplanted, more of them engraft; they then start producing insulin much more rapidly. These results, to be discovered in Nature Communications, would not only improve the success of cell transplants, but also offer new perspectives for other types of transplants, including stem cell transplantation.
Today, islet transplantation is one of the last-chance options for patients with a particularly severe form of type 1 diabetes. The islets are removed from a donor’s pancreas, isolated and then re-injected into the patient’s liver. “The procedure is well controlled – about fifteen patients benefit from it every year in Switzerland – but nevertheless complex, says Ekaterine Berishvili, a researcher in the Department of Surgery at UNIGE Faculty of Medicine, who led this work. Many of the islets die along the way. It often takes several donors to treat one person, whereas we are in desperate need of donors.”
Placental cells to help grafts
To improve the success of islet transplantation and the survival of transplanted cells, researchers in Geneva have sought to create new, more robust islets that would withstand the stress of transplantation better than natural islets. To do this, they came up with the idea of adding amniotic epithelial cells, taken from the wall of the inner placenta membrane, to the pancreatic cells. “These cells, very similar to stem cells, are already used in other therapies, such as corneal repair for example,” says Thierry Berney, Professor in the Department of Surgery at UNIGE Faculty of Medicine and Head of HUG Transplant Division, who co-directed this work. “In our case, we found that they can promote the function of pancreatic cells, which is to produce hormones according to fluctuations in sugar levels.”
First step, in vitro: the addition of amniotic epithelial cells allowed the cell clusters to form regular spheres, indicating better intracellular communication and connectivity. Second step in vivo: the scientists transplanted their “super-islets” of Langerhans into diabetic mice, which quickly began to produce insulin. “Even with few cell clusters, our super islets adapted very well to their new environment and quickly became vascularized,” says Fanny Lebreton, a researcher in the Department of Surgery at UNIGE Faculty of Medicine and the first author of this work. A good vascularization is indeed the key element of any transplantation: it allows to supply the new organ with oxygen and nutrients and guarantees their survival. In addition, the artificial islets quickly began to produce insulin.
Improving oxygenation and protecting islets
Amniotic epithelial cells are thus essential to islet survival and seem to act on two vital elements: the lack of oxygen, which usually kills a large number of transplanted islets, and the modulation of the host immune system to limit the risk of rejection. “In any transplant, the first step is to lower the recipient’s immunity to limit the risk of rejection, says Ekaterine Berishvili. Amniotic epithelial cells have the unique characteristic of protecting the foetus, which is also a “non-self,” from attacks by its mother’s immune system. We believe that the same mechanism is at work to protect the grafts.” The protective mechanism, observed here on cell transplants, could also take place in other types of transplants or even in xenotransplantation – where non-human cells or organs are transplanted into humans.
These discoveries now need to be confirmed on human subjects. Since the use of amniotic epithelial cells is already common in other clinical settings without adverse side effects, this could be done relatively quickly. An important hope for all those awaiting a transplant.
###
This work was supported by the Swiss National Science Foundation, the Fondation Privée des HUG, the Fondation Romande de Recherche sur le Diabète and the European Foundation for the Study of Diabetes, as well as by the Juvenile Diabetes Research Foundation.
Media Contact
Ekaterine Berishvili
[email protected]
41-223-795-113
Related Journal Article
http://dx.




