Credit: Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development at The Rockefeller University
Given the amount of wear and tear it’s subjected to on a daily basis, the skin has a phenomenal ability to replenish itself. Spread throughout it are small reservoirs of stem cells, nested within supportive microenvironments called niches, which keep a tight rein on this repair process. Too much tissue might cause problems like cancer, while too little might accelerate aging.
Until now, scientists were uncertain whether the stem cells themselves could instruct other stem cells to form new skin by reshaping their niche. But new research in Science, led by Elaine Fuchs, the Rebecca C. Lancefield Professor, indicates that stem cells can indeed influence tissue regeneration. The study identifies a molecular coordination tool used by stem cells to signal across niches.
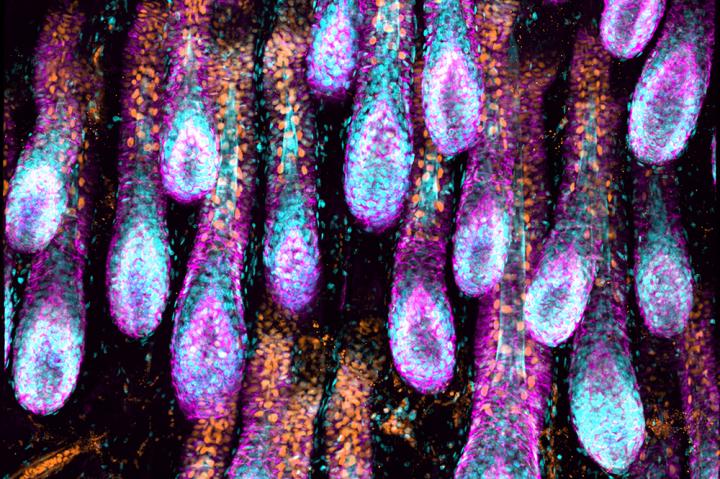
The researchers also discovered a new component of the niche: a specialized type of vessel called lymphatic capillaries, which transport immune cells and drain excess fluids and toxins from tissues. These capillaries form an intimate network around the stem cell niche within each hair follicle, the study showed, thereby interconnecting all its niches.
“By turning the skin completely transparent,” says postdoctoral fellow Shiri Gur-Cohen, “we were able to reveal the complex architecture of this network of tubes.”
Hair-follicle stem cells control the behavior of lymphatic capillaries by secreting molecules that act as an on-off switch for drainage, the scientists found, enabling them to control the composition of fluids and cells in the surrounding locale and ultimately synchronize regeneration across the tissue.
“The involvement of the lymphatic system in this process is a new concept,” says Fuchs, “and might potentially provide new therapeutic targets for lymph-related conditions such as wound-healing defects and hair loss.”
###
Media Contact
Katherine Fenz
[email protected]
212-327-7913
Original Source
https:/
Related Journal Article
http://dx.




