Rice scientists disentangle protein-nanoparticle interactions, suggest path to drug-sensing tool
Credit: Rashad Baiyasi/Rice University
HOUSTON – (Sept. 26, 2019) – It’s always good when your intuition turns out to be right, but scientists at Rice University studying proteins and particles were more “right” than they expected.
Rice chemists Christy Landes and Stephan Link and lead author and Smalley-Curl Postdoctoral Fellow Qingfeng Zhang reported this week in Science that bovine serum albumin (BSA), a standard-issue protein in nano-bio lab experimentation, is prone to pushing gold nanorods into right-handed chiral assemblies — while producing chiral plasmonic signals to match.
The result was a surprise to the researchers who set out to untangle the mysterious interactions in mixtures of BSA and gold nanorods: the aggregation of metallic nanoparticles into chiral assemblies, protein chirality, and the resulting plasmonic properties. Light triggers mixtures of particles and proteins to scatter polarized light, but until now researchers haven’t known which interactions within the mixture were responsible for the signal and, most important for future sensing applications, whether they could be refined.
The work hints it may become possible to sense the handedness, or chirality, of single proteins, a potential boon for pharmaceutical companies that require drug purity. A molecule with the correct chirality can save a life, while the same molecule of the opposite chirality can be highly toxic.
The Rice experiments revealed multilevel chirality in the way BSA proteins prompted the 100-nanometer-long particles to align and in how the particles’ plasmons responded to light in the much smaller proteins’ presence. (Plasmons are resonating electron waves that ripple along the surface of a metal particle when triggered by light.)
The response was measured as circular dichroism (CD), which is a way to measure if a light wave’s electric field rotation has a preferential interaction with material in either a clockwise (right) or counterclockwise (left) direction.
CD signals are usually weak, but still help characterize the average conformation of protein ensembles. In the Rice study, plasmons acted as antennas to amplify CD from the surface-adsorbed chiral proteins, shifting the signal from the ultraviolet to the visible, referred to as plasmon coupled-CD.
Link said plasmon-coupled CD had previously been observed for mixtures of single nanoparticles, aggregates and chiral molecules, but no study until now revealed whether all the molecules and nanoparticles contributed equally to the signal.
They don’t in this case. The study revealed that only aggregated nanorod-protein complexes produce a CD signal, caused both by proteins in the gaps between nanoparticles and by chiral nanoparticle assemblies.
It was a complete surprise, Landes said, that proteins directed the assembly of chiral nanorods in such a way that the handedness of the assembly matched the handedness of the proteins. “We were trying to test a hypothesis about the origin of the chiral signal that other people have reported in studies of nanoparticle ensembles,” she said. “Is it from chiral nanostructures? Is it from the protein? And we found that it’s both.
“In addition, the protein biomolecule with a preferential handedness induces that handedness in much larger nanorod aggregates,” Landes said. “That was the discovery we just didn’t expect.”
Link explained nanorod chirality is a matter of positioning. “Two perpendicular nanorods are achiral,” he said. “If they’re parallel, they’re achiral. But if they’re aligned at other angles, they’re chiral. The difficulty was in designing an experiment to determine where the CD comes from when you have mixtures of proteins, nanorods and achiral and chiral aggregates.”
Using a new technique developed in the Link lab called single-particle circular differential scattering (CDS) spectroscopy, the researchers confirmed that only aggregated nanorod-BSA complexes exhibited chirality. When the aggregates were excited with visible light, the antenna effect of the plasmonic hot spots amplified the normally weak chiral response of proteins in the inter-particle gaps.
Key to their success, Landes said, was a custom imaging program by Rice graduate student and co-author Rashad Baiyasi that let them identify single particles and small aggregates among the nanoscale samples and then relate the spectroscopy to the hot spots and preferential ordering.
Spring sabbaticals for both Landes and Link turned out to be perfect timing for the project as well, as they got the attention of co-author Jennifer Dionne, director of the Stanford Photonics Research Center and a specialist in cryogenic electron microscopy. Dionne showed that freezing the particle-protein solutions would allow the researchers to directly view how the particles aligned in 3D.
That helped eliminate any uncertainty that flattening the chiral assemblies on a surface was changing the signal.
In another experiment, the researchers replaced BSA with dissolved table salt to see how the particles reacted. They found the nanorods would aggregate, but into a mixture of achiral and chiral arrangements having the same amount of left- and right-handed species without an overall preferred handedness, and therefore without an ensemble CD signal. “It confirmed that BSA biases the formation of a certain handedness of nanostructures,” Link said. “We still don’t know why or how general this phenomenon is.”
Landes said the researchers are “about 14 steps” from judging the chirality of a single protein. If that’s possible at all, she said the Rice discovery may provide the only path toward label-free sensing of single protein conformations. That has value beyond measure for pharmaceutical chemists who struggle to create batches of proteins with a particular handedness, a critical factor in drug design.
“The ultimate dream has two parts: One is that we can detect conformations of single proteins dynamically, and the other is that we can do it inside living tissue,” Landes said. “There’s no way ever that you could use (invisible) ultraviolet light to do that. The only way to image inside something alive — a cell or an animal — would be to shift that light to the red, as we’ve done in these experiments.”
###
Additional co-authors of the paper are graduate students Seyyed Ali Hosseini Jebeli, Lauren Warning and Lauren McCarthy and assistant research professor Hua Guo of Rice; Rice alumni Taylor Hernandez and Kyle Smith, now of Draper Laboratory, Houston, Texas; and graduate student Alan Dai and senior research scientist Dong-Hua Chen of Stanford University.
Dionne is an associate professor of materials science and engineering at Stanford. Landes is a professor of chemistry, of electrical and computer engineering and of chemical and biomolecular engineering. Link is a professor of chemistry and of electrical and computer engineering.
The Robert A. Welch Foundation, the National Science Foundation, the Smalley-Curl Institute, the Gordon and Berry Moore Foundation, the SLAC National Accelerator Laboratory and the Stanford School of Medicine supported the research.
Read the abstract at https:/
This news release can be found online at news.rice.edu.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Video:
https:/
Video produced by Brandon Martin/Rice University
Related materials:
Landes Research Group: https:/
Link Research Group: https:/
The Dionne Group: http://dionne.
Rice Department of Chemistry: https:/
Wiess School of Natural Sciences: https:/
Images for download:
https:/
Rice University postdoctoral fellow Qingfeng Zhang works in the laser lab where he and his colleagues discovered the unusual chiral interplay between gold nanorods and bovine serum albumin proteins, the topic of a paper in Science. Their work revealed the proteins prompt gold nanorods to form right-handed chiral assemblies while producing chiral plasmonic signals to match. The phenomenon could lead to a technique that lets researchers determine the chiral purity of proteins critical to drug design. (Credit: Jeff Fitlow/Rice University)
https:/
Rice University postdoctoral fellow Qingfeng Zhang and his colleagues revealed in Science their discovery of multilevel chirality in the way bovine serum albumin proteins prompt the 100-nanometer-long particles to align and in the signal they return via circular differential scattering spectroscopy. (Credit: Jeff Fitlow/Rice University)
https:/
A team of Rice University researchers, with collaborators at Stanford University, set out to untangle the interactions in mixtures of proteins and gold nanorods and found that bovine serum albumin triggers multilevel chirality in the complexes. From left: front, Lauren Warning, Qingfeng Zhang and Rashad Baiyasi, and rear, Stephan Link, Seyyed Ali Hosseini Jebeli, Lauren McCarthy and Christy Landes. (Credit: Jeff Fitlow/Rice University)
https:/
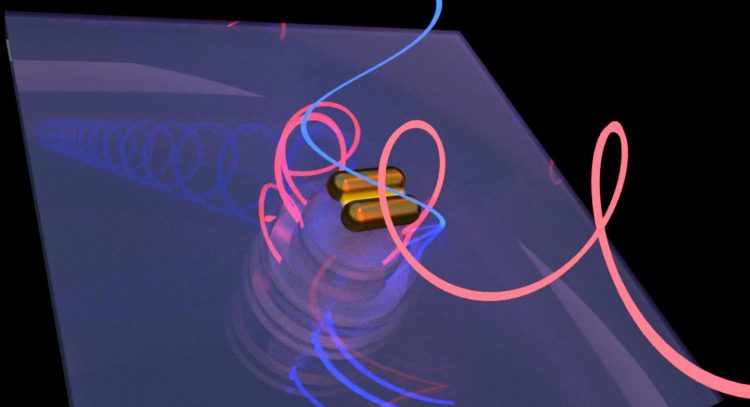
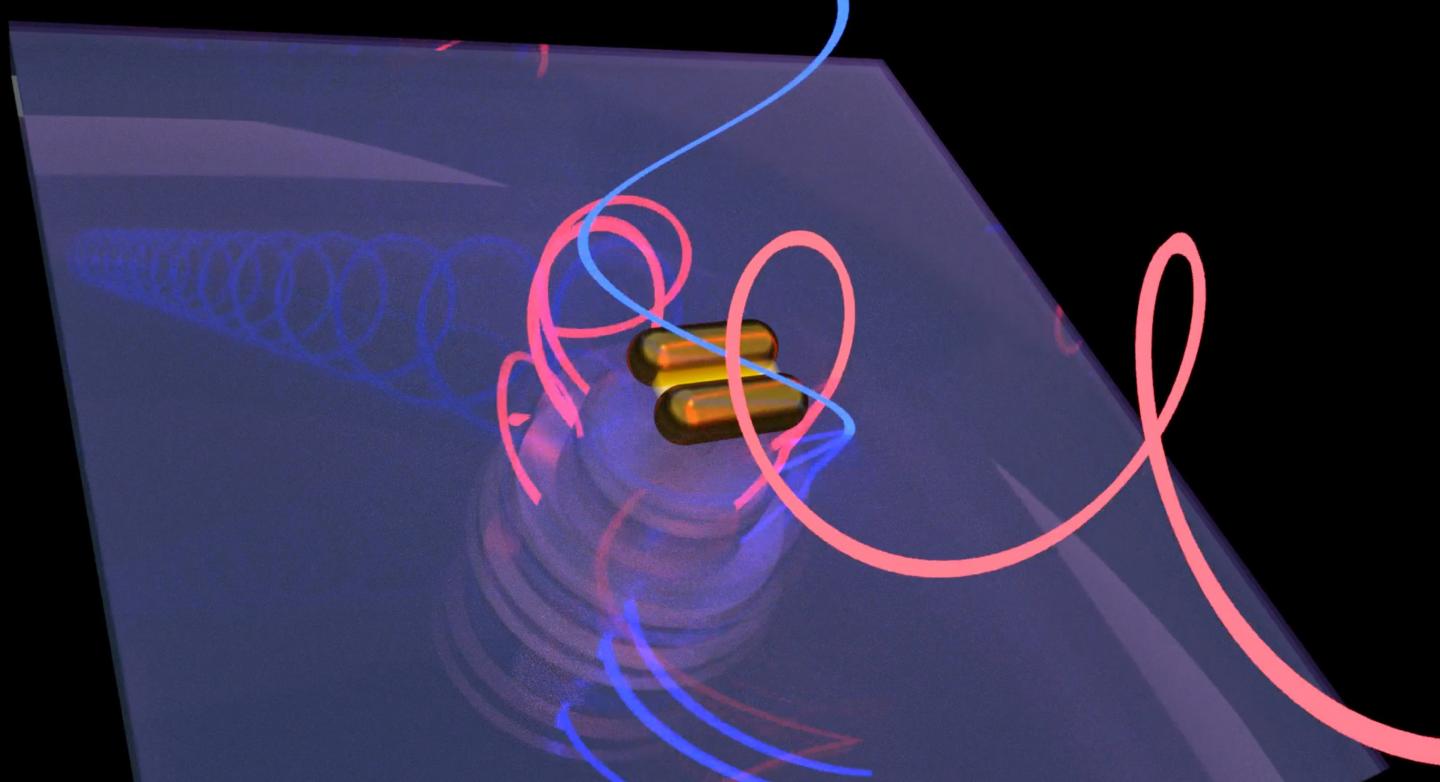
Work by scientists at Rice and Stanford universities revealed that bovine serum albumin proteins prompt gold nanorods to form right-handed chiral assemblies while producing chiral plasmonic signals to match. The phenomenon could lead to a technique that lets researchers determine the chiral purity of proteins critical to drug design. (Credit: Rashad Baiyasi/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Media Contact
Mike Williams
[email protected]
Related Journal Article
http://dx.