Credit: Laboratory of Membrane Biology and Biophysics at The Rockefeller University
In recent decades, treatment options for people with cystic fibrosis have improved dramatically. The newest drugs, known as potentiators, target a protein called cystic fibrosis transmembrane conductance regulator, which is mutated in people with the disease. Yet, while these medications can help some people with CF, they are far from perfect. Moreover, researchers haven’t been able to figure out how the drugs actually work–until now.
A new study by Rockefeller scientists characterizes, for the first time, the interaction between potentiators and the protein they target at atomic resolution. The research, described in a recent report in Science, shows that two distinct compounds act on the same protein region–a finding that points to strategies for developing more effective drugs.
Finding the hotspot
The cystic fibrosis transmembrane conductance regulator (CFTR) is a channel that, when open, allows chloride ions to move in and out of cells. When CFTR is mutated, ions cannot flow freely, leading to changes in the make-up of mucus lining internal organs. These changes can be particularly dangerous in the lungs where they cause thick mucus to accumulate, often leading to impaired breathing and persistent infections.
Potentiators are used to increase the flow of ions through CFTR, ameliorating some symptoms of cystic fibrosis (CF). Currently, only one such drug, known as ivacaftor, is on the market; another, called GLPG1837, is now in development.
“Ivacaftor can improve lung function by about ten percent. It can help a lot, but it’s not a cure and not everybody responds to it,” says Jue Chen, the William E. Ford Professor. “So there’s a lot of interest in developing new potentiators.”
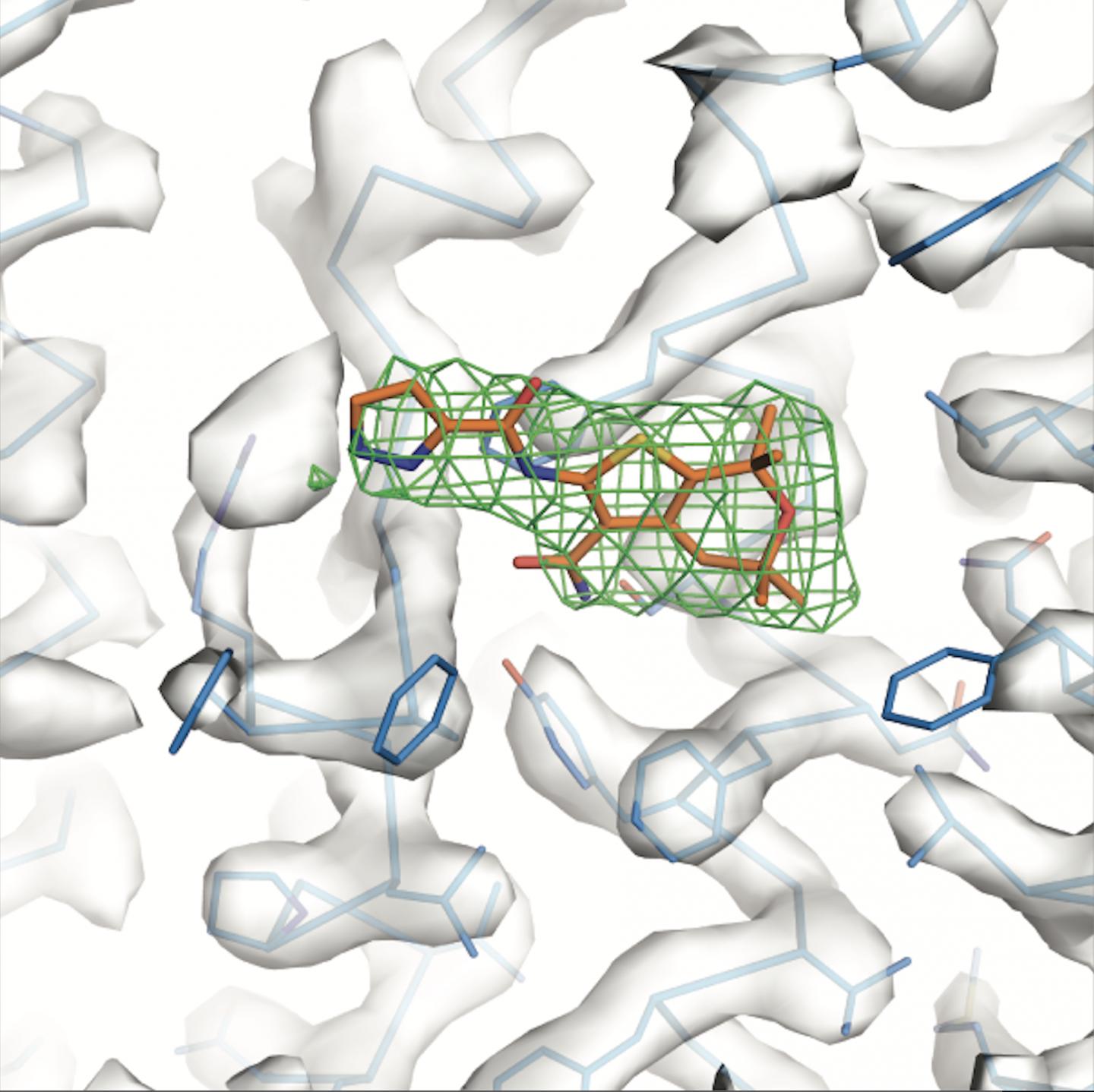
Pursuing this goal, Chen and her colleagues investigated how existing potentiators work. They used cryo-electron microscopy–a technique that beams electrons at a frozen specimen to reveal protein architecture at an atomic level–to study the structure of CFTR attached to either ivacaftor or GLPG1837. Somewhat surprisingly, the researchers found that the two drugs bind to the exact same spot on the protein.
“These compounds are developed by two different companies and have very different chemical properties. But they manage to make their way to the same site,” says Chen. “That tells us that this is a very sensitive, very important region of the protein.”
Better drugs, more access
Upon analyzing the “hotspot” where the two potentiators bound, the researchers noticed a peculiar feature: this area contained unwound loops inside the membrane that signify a flexible structure. And this flexibility, the researchers realized, serves a practical function.
“The region we identified, it turns out, works as a hinge that swings open to allow ions through the channel–so its structure needs to be flexible,” says Chen. “The compounds we studied bind to that very region, locking it into a channel-open conformation to improve ion flow. That’s how they work.”
With this knowledge, the researchers hope to craft compounds that directly target the hinge and do an even better job at keeping the ion channel open. And as Chen and her colleagues work toward the development of new drugs, she encourages other researchers to do the same. This kind of competition, she hopes, will drive down the cost of potentiators, making the medication available to a much larger portion of patients.
“We put our original data online and welcome anyone to use it,” says Chen. “Because if more researchers use it, more treatment options will become available, prices will drop, and more people will be helped.”
Reflecting on this breakthrough study, Chen acknowledges the work of David C. Gadsby, who passed away this March. The Patrick A. Gerschel Family Professor Emeritus and head of the Laboratory of Cardiac and Membrane Physiology, Gadsby’s early work on CFTR laid the groundwork for much of Chen’s research.
“He did a series of beautiful functional studies of CFTR, and he was a source of inspiration and knowledge,” she says. “It’s a pity he didn’t live to see it. We dedicate this study to him.”
###
Media Contact
Katherine Fenz
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




