Credit: Yasuhiro Kohsaka Ph D., the Research Initiative for Supra-Materials, Shinshu University
Before you read this, look around your room. How much of your surroundings are made of plastic? The chair that you sit on, the desk, the casing on your computer and monitor, the pen you use, the carpet, the shoes you wear, your clothes, your bag, the soda bottle you sip from, the furniture, the walls and even the plumbing- how many items can you identify that are plastic? Depending on where you reside, the majority of the things around you might be made of different types of plastic. Now, if you’re outside, various parts of your car, the buses and trains, even the interior of airplanes are mostly plastic. Currently there are not many methods to recycle plastics efficiently without compromising quality. So, it shouldn’t come as a surprise that not a day goes by without news of microplastics in our oceans and possibly in our food supply.
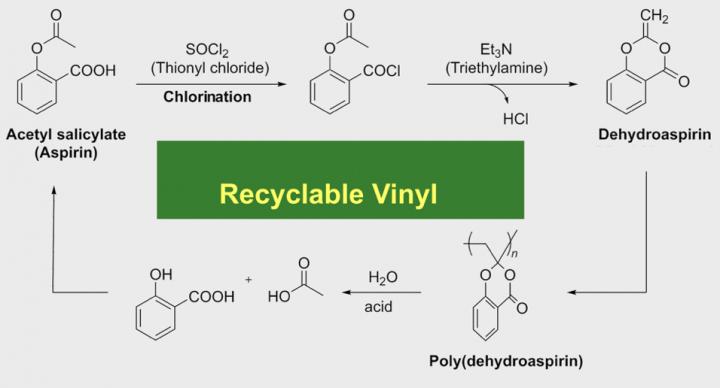
A beacon of hope was recently lit at Shinshu University where Professor Yasuhiro Kohsaka and his graduate student Akane Kazama discovered acid hydrolysis of a vinyl polymer broke down into salicylic acid and acetic acid. These acids form aspirin through some reactions. Vinyl is the second most common plastic in the world today. Previous recyclable vinyl had been too unstable to work with at room temperature, and was not suitable for practical use.
The team at Shinshu plan to study the reaction mechanism in-depth which they hope will provide insight into real world applications for recyclable vinyl polymers. If it can become cost effective to recycle vinyl on an industrial scale, we will be one step closer to solving the global plastic waste problem.
###
Further information: Polymer Chemistry 2019, DOI: 10.1039/C9PY00474B
Media Contact
Hitomi Thompson
[email protected]
Related Journal Article
http://dx.




