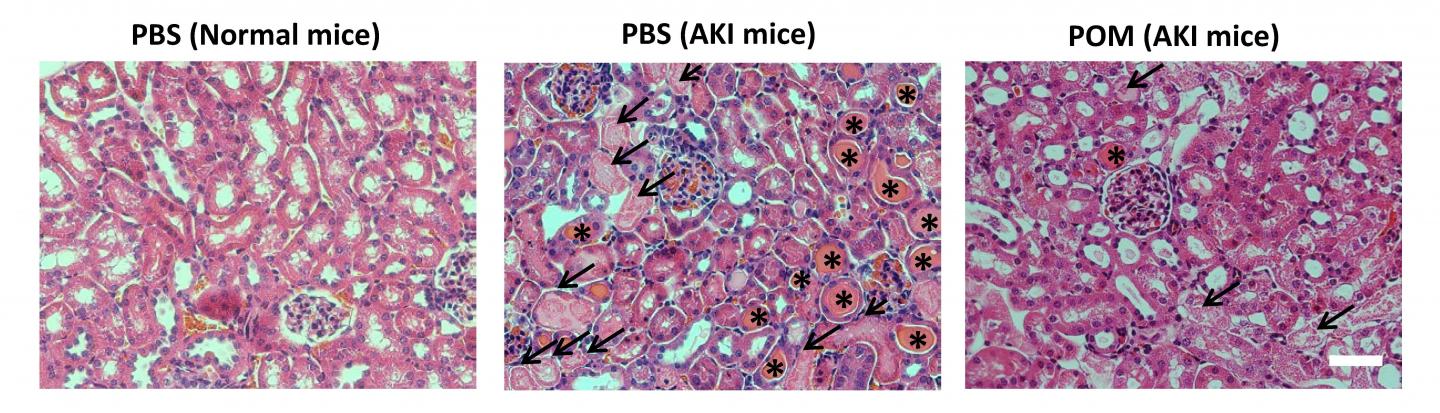
Credit: Credit: D. Ni and W. Cai, et al., Department of Radiology, University of Wisconsin-Madison, Madison, WI.
ANAHEIM, CA (Embargoed until 9 a.m. PDT, Tuesday, June 25, 2019) – Acute kidney injury (AKI) often complicates the treatment outcomes of hospitalized patients, resulting in dangerous levels of toxic chemicals accumulating in the blood and causing numerous deaths annually. Currently, only supportive treatment is available for AKI, but two related research studies presented at the 2019 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging offer hope for effective treatment and prevention.
Reactive oxygen species (ROS), free radicals that trigger oxidative stress and inflammation, are believed to induce AKI. Both studies used mouse models to test new potential AKI therapies that target ROS.
The first study developed tiny nanoclusters with antioxidative properties that can pass through the kidneys’ glomerular filtration barrier to treat AKI.
“In this study, ultra-small sized molybdenum-based polyoxometalate (POM) nanoclusters with preferential renal uptake were found to act as a novel type of nano-antioxidant to efficiently alleviate clinical symptoms of AKI mice,” explains Dalong Ni, PhD, at the University of Wisconsin-Madison.
POM radiolabeled with zirconium-89 (89Zr) was intravenously injected into AKI mice. Longitudinal PET imaging was then performed at various time points to monitor the renal accumulation in vivo. The therapeutic value was evaluated by dynamic positron emission (PET) scans with gallium-68 (68Ga)-EDTA, blood urea nitrogen and creatinine measurements, H&E staining of kidney tissues, and biomarkers detection.
Ni reports, “The role of POM nanoclusters acting as antioxidants was confirmed both in vitro and in vivo, which was attributed to the readily variable valence state of molybdenum ions. Kidney transplantation and supportive therapies, such as rehydration and dialysis, are frequently required for patients with AKI. The protective effect of POM nanoclusters against AKI in living animals suggests exploring their use for the treatment of AKI patients.”
He adds, “POM clusters could be synthesized in an easy, fast, and large-scale process, and they are mainly excreted through the kidneys (like most clinically-used imaging agents), making them highly biocompatible and reducing the potential toxic effects on patients.”
The second study, also from the University of Wisconsin-Madison, uses extremely small nanomaterials as well. It demonstrates that selenium-doped carbon quantum dots can both treat and prevent AKI by localizing in the kidneys and eliminating ROS.
For the study, carbon quantum dots with high antioxidant capacity were prepared by doping selenium (SeCQDs) through a hydrothermal treatment. Molecular imaging provides the most effective method for investigating the biodistribution of nanomaterials, so PET imaging of SeCQDs was preformed to evaluate biodistribution using 89Zr after functionalizing with deferoxamine. Results of the SeCQDs treatment of the mice were compared with those from administration of an equivalent dose of amifostine, an FDA-approved drug for AKI therapy.
“The PET imaging results revealed that SeCQDs possess fascinating nano-bio interactions,” explains Zachary Rosenkrans in the Pharmaceutical Sciences Department at the University of Wisconsin-Madison. “Surprisingly, we found that the administered dose almost entirely accumulated in the kidneys or was rapidly excreted in the urine, with little present in the liver or other organs. Results similar to this have rarely been reported.”
The high accumulation of SeCQDs in the kidneys proved a highly effective therapy for the treatment of AKI that was induced using 50 percent glycerol, as well as prevention of AKI from cisplatin due to its antioxidant properties. In the same animal models, amifostine was unsuccessful in treating AKI.
Rosenkrans points out, “Due to this, we were able to demonstrate the advantages of utilizing nanomaterials compared to small molecules for renal disease treatment–in this case, AKI. This could most directly benefit the one in five hospitalized patients that experience AKI.”
Excited about next steps, he adds, “This work could lead to guidelines to tailor nanoparticles to target and be retained in the kidneys, which has transformative potential to treat renal diseases. This may provide new avenues for more effective delivery of drugs to kidney cancer (or other kidney diseases, such as glomerulonephritis) and thus more efficacious therapies.”
Both abstracts were presented at SNMMI’s 66th Annual Meeting, June 22-25, Anaheim, CA.
###
To schedule an interview with the researchers, please contact David Harrison at (410) 804-1728 or [email protected]. All 2019 SNMMI Annual Meeting abstracts can be found online at http://jnm.
About the Society of Nuclear Medicine and Molecular Imaging
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging, vital elements of precision medicine that allow diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes.
SNMMI’s more than 16,000 members set the standard for molecular imaging and nuclear medicine practice by creating guidelines, sharing information through journals and meetings and leading advocacy on key issues that affect molecular imaging and therapy research and practice. For more information, visit http://www.
Media Contact
David Harrison
@SNM_MI
410-804-1728