Novel method to engineer large multi-antibody-like nanostructures using DNA nanotechnology; the results demonstrate the potential for assembly of multiple proteins and also other materials to enhance properties of traditional therapies
Collaboration between Novo Nordisk and Professor Kurt Gothelf’s laboratory at Aarhus University yields novel method to engineer large multi-antibody-like nanostructures using DNA nanotechnology
Novo Nordisk and the Faculty of Science and Technology at Aarhus University have committed to a strategic alliance to further develop excellent research within protein and peptide-based drug development.
Today, a step towards this goal has been taken in the publication of a new efficient method for connecting small pieces of proteins attached to short strings of DNA to antibodies. The method is developed by a research team at Novo Nordisk and Kurt Gothelf’s research group (Gothelf Lab). The work is also performed in the framework of the Center for Multifunctional Biomolecular Drug Design which started last year under the Novo Nordisk Foundation Challenge Programme.
Mimicking naturally occurring molecules and components, like antibodies, can serve as a powerful tool in studying biological mechanisms. Here DNA nanotechnology is utilized to integrate protein function and DNA structures, where nucleic acids (DNA building blocks) are used as biological engineering materials rather than as the carriers of genetic information in living cells. The advantages of DNA nanostructures are that the production is scalable and can be made in the dimensions relevant for clinical use and for scientific experiments with cells. Connecting proteins to DNA structures adds complex functionalities, which can provide the structures with the ability to act as drugs, extend the lifespan of the molecules or direct the structures towards specific molecules.
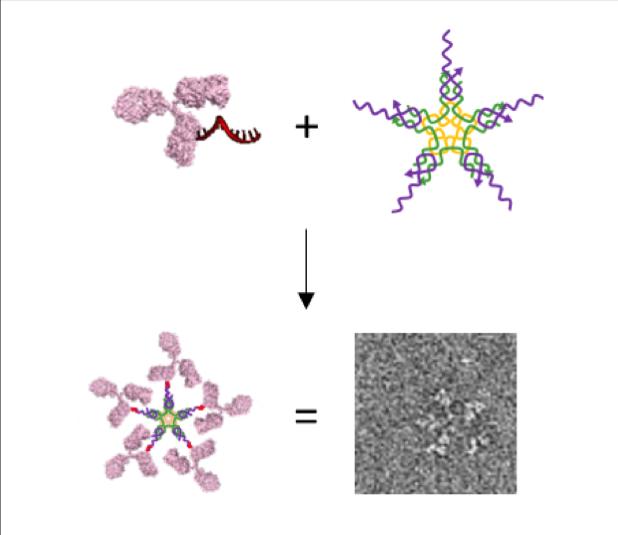
Here the researchers show the development of a new method for connecting a piece of DNA to a specific spot on Immunoglobulin Gs (IgGs), which are the most prevalent antibodies in our blood stream. The connection (labeling) is directed by a small piece of protein (peptide), with affinity to a specific spot on the antibody, which positions a single piece of double-string DNA. The peptide along with half of the DNA double-string is easily removed afterwards, and leaves a single string of DNA chemically bound to the antibody. This makes it possible to connect the DNA-antibody conjugate to structures with a complementary DNA string attached.
The DNA-conjugates have been exploited in forming an IgM-like nanostructure, which is a large star-like pentameric DNA nanostructure made by assembling five DNA-antibody conjugates.
“It is very difficult to link DNA to an antibody in a specific and efficient manner and I am very excited about this work since it offers a solution to exactly that challenge. This is important since it allows us to assemble multiple complex biomolecules in a very well-defined manner just by mixing the components as demonstrated for the pseudo-IgM. The DNA strands are designed to control the self-assembly. The work has only been possible through a wonderful collaboration with Novo Nordisk, where they have contributed with their strong expertise in peptides and the researchers at iNANO including my collegue Professor Jørgen Kjems’ group have contributed with bioconjugation and nanocharacterization. Everything happened in close collaboration in particular by the joint PhD student and first author Thorbjørn Nielsen, who did a fantastic job“, says Professor Kurt Vesterager Gothelf (Aarhus University).
“I feel that Novo Nordisk has only just started the journey into DNA-modified protein drugs with Aarhus University. By combining our expertise in protein and peptide engineering with the unique DNA technologies developed by Kurt Gothelf’s Laboratory, the project team led by senior scientists Emiliano Cló and Anne Louise Bank Kodal has creatively developed a novel molecular scaffold with promising opportunities for therapeutic applications. We hope that the developed technologies can help us understand critical protein-protein interactions of drug targets currently explored at Novo Nordisk. A virtually unexplored scientific field is evolving in front of us, and we cannot predict where it will take us, but opportunities are clearly huge. Needless to say; we are overly excited to collaborate with Aarhus University on DNA-modified proteins in the hunt for new biopharmaceuticals, now and in the future“, says Director Thomas E. Nielsen, Research Chemistry, Novo Nordisk A/S.
###
This work was financially supported by the Innovation Fund Denmark, Novo Nordisk Foundation (CEMBID) and the National Danish Research Foundation (CDNA and CellPat).
The research has been carried out by scientists from Interdisciplinary Nanoscience Centre (iNANO), Department of Chemistry and Department of Molecular Biology and Genetics and in collaboration with Novo Nordisk A/S. Professor Kurt Vesterager Gothelf has been in charge of the research team behind the study.
Read about the study in Angewandte Chemie Int. Ed.:
Nielsen, T.B.; Thomsen, R. P.; Mortensen, M. R.; Kjems, J.; Nielsen, P. F., Nielsen, T. E.; Kodal, A. L. B.; Clo, E.; Gothelf, K. V. Peptide-Directed DNA-Templated Protein Labelling for The Assembly of a Pseudo-IgM. Angew Chem Int Ed Engl. 2019. doi: 10.1002/anie.201903134
.
For further information, please contact:
Professor Kurt Vesterager Gothelf
Department of Chemistry and Interdisciplinary Nanoscience Center
Aarhus University
[email protected]
Media Contact
Kurt Vesterager Gothelf
[email protected]
Original Source
http://inano.
Related Journal Article
http://dx.



