Credit: Kanazawa University
[Background]
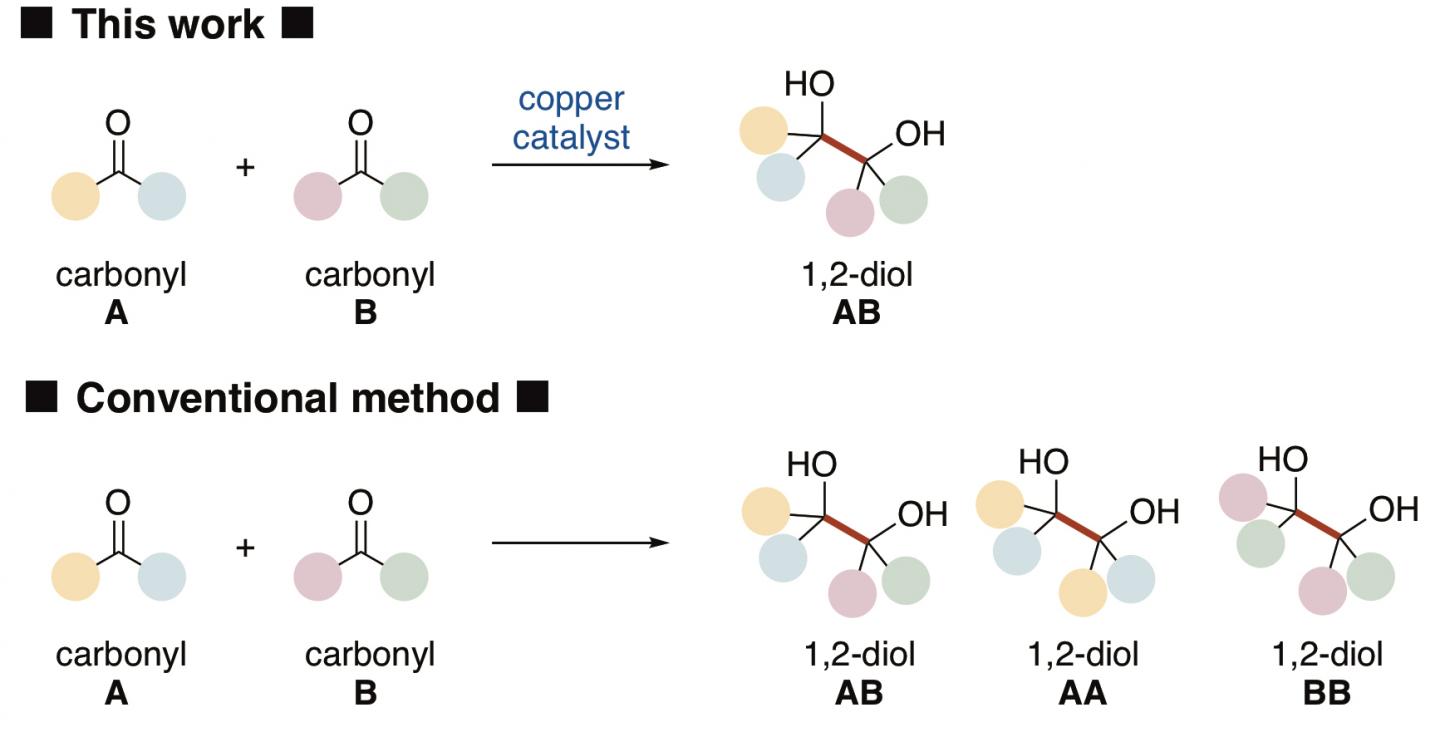
1,2-Diols*1) are common scaffolds in many pharmaceuticals, agrochemicals, and natural products. The most direct method of synthesis would be if two easily obtainable carbonyl*2) compounds as starting materials would be converted into a 1,2-diol by carbon-carbon bond formation between the two compounds by reductive coupling. However, since conventional methods need very harsh conditions for such a reaction, it is difficult to distinguish between the two carbonyls, e.g., A and B, and such a reaction would give three species of 1,2-diols, i.e. AB, AA and BB (Figure 1). This means that a complex procedure to separate three species of 1,2-diols is necessary.
[Outline of research results]
The present research team of Kanazawa University, including two students, was successful in selectively synthesizing one species of 1,2-diol from an aldehyde and a ketone, both of which are carbonyl compounds, as the starting materials (Figure 2). The key to the success was a newly developed copper catalyst that could distinguish between the two different carbonyl compounds, activate each compound in a different manner and connect two different compounds to selectively yield a 1,2-diol. Copper is abundantly available and since its toxicity is not so high it is considered to be friendly to both humans and the environment. It was important to use N-heterocyclic carbene*3) as the ligand*4) to the copper catalyst.
More specifically, the copper catalyst makes the following two processes, 1 and 2, take place sequentially (Figure 3).
- After addition of silylcopper species to an aldehyde and the subsequent transfer reaction, an active copper species equivalent to a hydroxycarbanion*5) is catalytically generated.
- The active copper species thus formed is captured by a ketone.
By using a chiral*6) N-heterocyclic carbene as the ligand to the copper catalyst, the team has also succeeded in asymmetric synthesis*7) of chiral 1,2-diols.
[Future prospect]
In this study, the team has developed a new copper catalyst that could distinguish between two different carbonyl compounds and form a carbon-carbon bond to connect them, yielding one species of 1,2-diol. This type of selective synthesis was known to be quite difficult; the present success should open up a way for organic synthesis of 1,2-diol chemicals. It is expected to lead to a rapid synthesis of pharmaceuticals, agrochemicals, and natural products of complex structure.
###
[Glossary]
*1) 1,2-Diol
A compound whose two vicinal carbon atoms have hydroxyl groups.
*2) Carbonyl
A functional group with a double bond between a carbon atom and an oxygen atom.
*3) Carbene
A carbene is a twofold coordination chemical species containing a neutral carbon atom with only six valence electrons.
*4) Ligand
A ligand is an organic molecule that binds to a central metal atom.
*5) Carbanion
A carbanion is a chemical species in which carbon bears a negative charge.
*6) Chiral
Chirality is a geometric property of some molecules and ions, and a chiral molecule/ion is non-superimposable on its mirror image, just like our right and left hands. A number of chiral molecules, enantiomers, can be found in the world of chemistry, while their constituting atoms and alignment are the same. Chiral molecules have similar chemical/physical properties while biological functions are sometimes quite different.
*7) Asymmetric synthesis
Asymmetric synthesis is a synthetic chemical reaction that selectively synthesizes one of the chiral molecules, usually a useful one.
[Special note]
This article is used for the Supplementary Cover of JACS February 27 (2019) issue.
Media Contact
Yuki Kashin
[email protected]
Related Journal Article
http://dx.




