Researchers use genetic manipulation techniques to highlight how the function of a protein can lead to neurodevelopmental delays
Credit: Katsuhiko Tabuchi, Shinshu University, Japan
Researchers have clarified, for the first time, the mechanism behind a very rare brain disorder called MICPCH (microcephaly, disproportionate pontine and cerebellar hypoplasia) syndrome in animal models. Information gleaned from this study could also inform research into other, more common neurological diseases such as mental retardation, epilepsy, and autism.
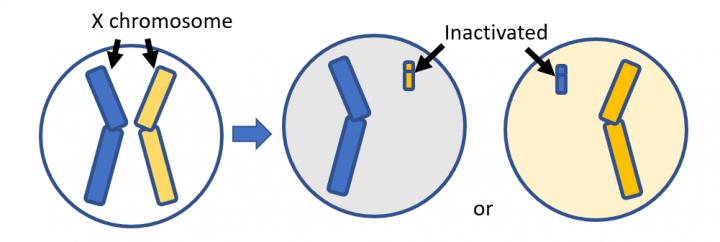
MICPCH has only affected a total of 53 females and seven males worldwide so far. It is characterized by several developmental symptoms including small head size, slowed growth, cognitive delays, epilepsy, seizures, vision and hearing problems, decreased muscle tone, and autism. MICPCH is linked to irregularities, or mutations, on the X-chromosome that eventually lead to the chromosome’s inactivation.
The study was published in the January 4th, 2019 edition of the journal Molecular Psychiatry.
Brain cells, or neurons, constantly communicate by sending messages to one another. There are two types of neurons in the brain: those that increase activity in other cells (excitatory neurons) and those that decrease it (inhibitory neurons). The mechanism behind keeping the balance between excitation and inhibition in the brain is very similar to that of a thermostat that is used to maintain a balanced temperature in a home. This mechanism is important because imbalances between excitation and inhibition can cause several serious disorders such as epilepsy and autism. One of the most important molecules that maintains the balance between excitation and inhibition is a protein found within the outer membrane of neurons, called the calcium/calmodulin-dependent serine protein kinase (CASK). Mutations in the gene that produce CASK therefore lead to several neurodevelopmental disorders such as mental retardation. A lack of the protein in the brain has been found to cause MICPCH syndrome.
“The aim of the study was to understand the pathophysiology of CASK-deficiency disorders in females, such as MICPCH syndrome, which are supposed to be influenced by X-chromosome inactivation,” said corresponding author Katsuhiko Tabuchi, a professor in the Department of Molecular and Cellular Physiology at the Institute of Medicine, Academic Assembly at Shinshu University in Nagano, Japan.
However, the details of CASK-deficiency consequences have thus far been difficult to study, as mice that completely lack the protein die before they are developed enough to study.
In order to understand the mechanism behind the CASK-deficiency, researchers at Shinhsu University in Japan and Kafr Elsheikh University in Egypt have used gene manipulation techniques that shut off the CASK gene through X-chromosome inactivation in female mice without lethal consequences.
They found that neurons that lack CASK have a disrupted excitation and inhibition balance. They also found that this is because of a decrease in concentration of a specific receptor on the membrane that receives signals from other neurons. When the receptor concentration was increased, the excitatory and inhibitory balance was restored again, leading the researchers to believe that the receptor plays a central role in the mechanism in CASK-deficient neurons.
In the future, the researchers hope to address the effects of a CASK-deficiency in even greater detail by looking at its effects on the neural circuitry. “We hope to highlight the effect of two different types of neurons in one brain as well as the pathophysiology of CASK-deficiency disorders at neural circuit levels,” professor Tabuchi adds.
###
The study was supported by a Grant-in-Aid for Young Scientists grant, several Grant-in-Aid for Scientific Research grants, several Grant-in-Aid for challenging Exploratory Research grants, the Foundation of Growth Science, the Japan Epilepsy Research Foundation, the Takeda Science Foundation, the Uehara Memorial Foundation, the Ichiro Kanehara Foundation, and the JST PRESTO Program: Development and Foundation of Neural Networks.
About Shinshu University
Shinshu University is a national university in Japan founded in 1949 and working on providing solutions for building a sustainable society through interdisciplinary research fields: material science (carbon, fiber, composites), biomedical science (for intractable diseases, preventive medicine), and mountain science. We aim to boost research and innovation capability through collaborative projects with distinguished researchers from the world. For more information, please see: https:/
Media Contact
Nobuko Imanishi
[email protected]
81-263-372-097
Related Journal Article
http://dx.




