Researchers present measures for reducing a risk of uterine cervical cancer that increased by the suspension of the Japanese government recommendation for the Human Papillomavirus (HPV) vaccination and for promoting HPV vaccination after its resumption
Credit: Osaka University
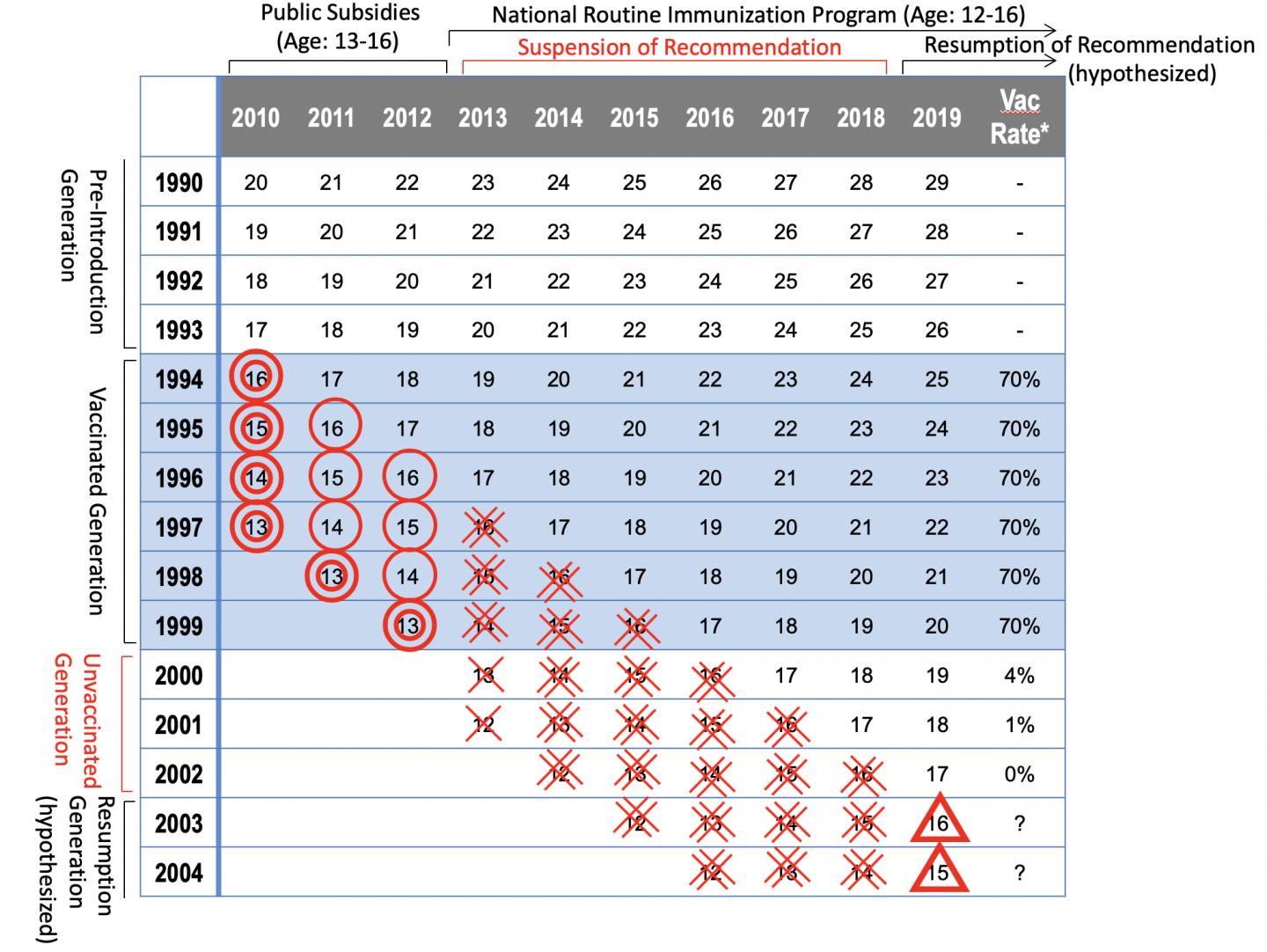
In Japan, HPV vaccination program started in 2010 and the HPV vaccine became a nationally recommended routine immunization for girls aged 12-16 years in April 2013. However, because cases of young girls with widespread pain and movement disorders after vaccination were reported in the media, in June 2013, Japan’s Ministry of Health, Labour and Welfare (MHLW) announced the suspension of its recommendation for routine HPV immunization.
Previous research at Osaka University showed that the HPV vaccination hiatus would increase the risk of HPV infection and future cervical cancer for girls who did not get vaccinated.
HPV vaccination is vital to reduce the risk of HPV infection and cervical cancer; however, no reports on countermeasures against expected challenges after resumption of HPV vaccination have been published.
Researchers at Osaka University compiled countermeasures against predictable challenges after resumption of HPV vaccination, publishing in the Lancet Oncology.
Every year, about 9,000 women are diagnosed with cervical cancer and 2,000 – 3,000 women die of the cancer. A major factor for the development of cervical cancer is infection with HPV, which is mainly transmitted through sexual contact.
This research group demonstrated two possible challenges after resumption of the MHLW’s recommendation for HPV vaccination:
1. Reducing the risk of cervical cancer that will increase by the suspension of the recommendation
2. Promoting HPV vaccination
The group discussed countermeasures against these challenges, making the following proposals for providing information and fostering public acceptance of the vaccine.
A. Easy access to immunization for women who are older than the normally targeted ages of 12-16 years and who were not vaccinated during the suspension of recommendation for HPV vaccination
B. Introduction of the nine-valent vaccine, which can prevent 80-90 percent of cervical cancer
C. Immunization for boys of the same ages as the targeted girls
D. Reducing health damage due to the suspension of recommendation for HPV vaccination by encouraging medical check-ups and cervical cancer screening
E. Promoting HPV vaccination again by using a behavioral economics approach
F. Providing media with correct information about the HPV vaccine
Dr. Yutaka Ueda says, “Resumption of the government recommendation for HPV vaccination will be insufficient to deal with the expected challenges. It’s necessary to reduce negative effects of the suspension of recommendation for HPV vaccination. We hope our proposals will reduce the development of cervical cancer in Japanese women, and, moreover, protect women’s health.”
###
The comment, “Beyond resumption of the Japanese Government’s recommendation of the HPV vaccine” was published in the Lancet Oncology at DOI: https://doi.org/10.1016/S1470-2045(18)30573-4.
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and now has expanded to one of Japan’s leading comprehensive universities. The University has now embarked on open research revolution from a position as Japan’s most innovative university and among the most innovative institutions in the world according to Reuters 2015 Top 100 Innovative Universities and the Nature Index Innovation 2017. The university’s ability to innovate from the stage of fundamental research through the creation of useful technology with economic impact stems from its broad disciplinary spectrum.
Website: https:/
Media Contact
Saori Obayashi
[email protected]
81-661-055-886
Original Source
https:/
Related Journal Article
http://dx.




