The healthy gut microbiome contributes to skeletal health, and disruption of this system with antibiotics leads to a pro-inflammatory immune response that dysregulates post-pubertal skeletal development
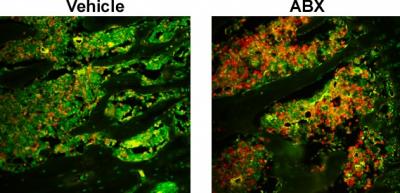
Credit: Dr. Chad Novince of the Medical University of South Carolina
Microbes are often seen as pathogens that cause disease and antibiotics have been used successfully to combat these foreign invaders. In reality, the picture is more complex. Most of the time we live in harmony with our commensal gut microbiota, which is the collection of microorganisms colonizing the healthy gut. Commensal bacteria regulate host biological functions, including skeletal health. Researchers at the Medical University of South Carolina (MUSC) studying osteoimmunology, the interface of the skeletal and immune systems, have examined the impact of disrupting the healthy gut microbiome with antibiotics on post-pubertal skeletal development. Their results, published online on the January 16, 2019 in the American Journal of Pathology, showed that antibiotic disruption of the gut microbiota induced a pro-inflammatory response that led to increased activity of osteoclasts.
“This report introduces antibiotics as a critical exogenous modulator of gut microbiota osteoimmune response during post-pubertal skeletal development,” says Chad M. Novince, D.D.S., Ph.D., assistant professor in both the Colleges of Medicine and Dental Medicine who studies the impact of the microbiome on osteoimmunology and skeletal development. “People have shown that antibiotics perturb the microbiota, but this is the first known study evaluating how that has downstream effects on immune cells that regulate bone cells and the overall skeletal phenotype. This work brings the whole story together.”
The post-pubertal phase of development is a critical window of plasticity that supports the accrual of approximately 40 percent of our peak bone-mass. Recent work by the Novince lab and others has shown that the gut microbiota contributes to skeletal health. To determine the impact of antibiotic perturbation of the gut microbiota in post-pubertal skeletal development, Novince worked with team members at MUSC and treated mice with a cocktail of three antibiotics. In collaboration with microbiome scientist Alexander V. Alekseyenko, Ph.D., associate professor in the Biomedical Informatics Center and founding director of the MUSC Program for Human Microbiome Research, they were able to show that antibiotic treatment led to major alterations in the gut microbiota, resulting in specific changes to large groups of bacteria.
“Having Dr. Alekseyenko as part of the team is a unique strength,” says Novince.
Following antibiotic disruption of the microbiota, the Novince lab examined the integrity of the skeletal system. Antibiotic-induced changes in the microbiota had little impact on cortical bone; however, there were significant changes to the trabecular bone, the type of bone that undergoes high rates of bone metabolism. While previous work looked at bone cell density in the whole skeleton upon antibiotic treatment, this work focused on the cellular details underlying bone maintenance. Bone metabolism is controlled through a balance of bone-resorbing (osteoclast) and bone-building (osteoblast) cells. Interestingly, there were no changes to the osteoblasts, while osteoclast cell number, size and activity were increased.
To determine what caused osteoclast activity to increase, the Novince lab assessed the levels of several osteoclast signaling molecules. They found that levels of pro-osteoclastic signaling molecules were increased in the circulation of antibiotic-treated animals, suggesting that increased osteoclast activity is the result of a specific immune response to a change in the microbiota.
The next major question was how antibiotics impact immune cells in the bone marrow environment.
“Our study is actually able to dive into specific adaptive and innate immune cell mechanisms within the bone marrow environment to show that there is an effect on the bone cells,” says Jessica D. Hathaway-Schrader, Ph.D., post-doctoral scholar and first author on this study.
Examination of immune cell populations in the bone marrow surprisingly revealed a significant increase in myeloid-derived suppressor cells (MDSCs) of antibiotic-treated animals. MDSCs are known to regulate the innate and adaptive immune response during various diseases, but have not been extensively studied in health. Additionally, antigen presentation and processing were suppressed in the bone marrow upon antibiotic treatment.
In summary, Novince’s group has shown that antibiotic disruption of the gut microbiota dysregulates communication between immune cells and bone cells. While the current study utilized a broad-spectrum antibiotic cocktail intended to grossly disrupt the composition of the gut bacteria, the results warrant further investigation. Future studies are aimed at incorporating an antibiotic regimen that better translates to human antibiotic treatments. These studies could lead to clinical trials aimed at defining the impact of specific antibiotics on the gut microbiome. This research would support developing non-invasive therapeutic interventions in the microbiome intended to prevent and treat skeletal deterioration.
###
About MUSC
Founded in 1824 in Charleston, The Medical University of South Carolina is the oldest medical school in the South. Today, MUSC continues the tradition of excellence in education, research, and patient care. MUSC educates and trains more than 3,000 students and residents, and has nearly 13,000 employees, including approximately 1,500 faculty members. As the largest non-federal employer in Charleston, the university and its affiliates have collective annual budgets in excess of $2.2 billion. MUSC operates a 750-bed medical center, which includes a nationally recognized Children’s Hospital, the Ashley River Tower (cardiovascular, digestive disease, and surgical oncology), Hollings Cancer Center (a National Cancer Institute designated center) Level I Trauma Center, and Institute of Psychiatry. For more information on academic information or clinical services, visit musc.edu. For more information on hospital patient services, visit muschealth.org.
Media Contact
Heather Woolwine
[email protected]
843-792-7669
Related Journal Article
http://dx.




