A West Virginia University biologist is studying why some animals can regenerate while others cannot and has identified the genes that play a role in the process.
A West Virginia University biologist is studying why some animals can regenerate while others cannot and has identified the genes that play a role in the process.
Christopher Arnold, assistant professor of biology at the WVU Eberly College of Arts and Sciences, will explore how genes establishing animal body plans — the structure of organs and tissues — also set the stage for regenerative abilities.
His research could provide insight into human development and disease, leading to enhanced understanding of factors underlying tissue regeneration and inspiring novel approaches to improving human health.
Arnold’s research is supported by a Maximizing Investigators’ Research Award from the National Institutes of Health.
According to Arnold, much about regeneration, which is the ability to repair and replace lost or damaged tissues, is still a mystery.
“We really don’t understand a lot about regeneration, and we have a bit of a misconception that regeneration is something super rare and that animals that can do it are out of the norm, particularly animals that regenerate their whole body,” Arnold said.
“While this amazing feat seems strange to us, highly regenerative animals are widely distributed throughout the tree of life and almost all branches have species that can regenerate their entire bodies. It is more likely that whole body regeneration is an ancestral trait lost in some animal lineages, including ours, but retained in many others. To put it simply, those animals aren’t weird because they can regenerate. We’re weird because we can’t.”
Regeneration may be more than just a response to injury. While injured animals do benefit from regeneration, the process doesn’t necessarily correlate with the tissues or the animals that are injured the most. For example, the human liver can famously regenerate to its original size even if 90% of the organ is removed.
But outside of surgery, a naturally occurring injury triggering this degree of regeneration would be fatal. There may be other reasons outside of the injury context underlying regeneration’s variable distribution across animals and their parts.
Hox genes, which are responsible for the patterning and evolution of a variety of body plans across the animal kingdom, may be key to understanding regeneration, Arnold said. Hox genes are highly conserved transcription factors that orchestrate the embryonic development of body plans of animals as different as insects and humans. Additionally, numerous human developmental disorders and diseases stem from Hox gene dysfunction, he said.
“We have a fundamental set of genes for patterning the body,” Arnold said. “Our work has identified unexpected roles for these genes in a highly regenerative animal. Maybe that isn’t an exception. Maybe these conserved body patterning genes function differently in highly regenerative versus poorly regenerative body plans. This could help us understand why some animals can still regenerate and others no longer can.”
Our understanding of Hox genes’ functions across the tree of life is incomplete as most research has been conducted on animals that are not highly regenerative, like mice and fruit flies. Until recently, their functions in animals with whole body regenerative abilities were largely unknown.
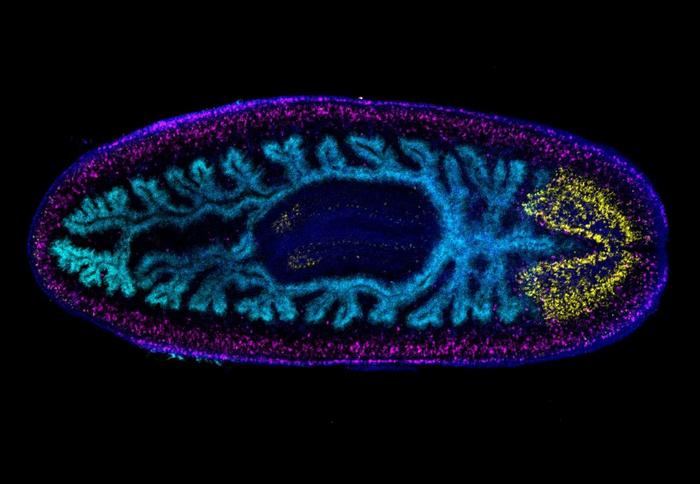
Arnold’s subject of choice is planaria, a flatworm that can regenerate its entire body, both after an injury and for asexual reproduction.
“Planaria can asexually reproduce by tearing off pieces of their body, and each one of those pieces will be able to regenerate into a full copy of the parent organism,” he said. “The planarian strain we work with originally came from a single animal collected in a fountain in Spain. It seeded millions upon millions of animals across labs in the U.S.”
Arnold has found that planarian Hox genes are required for both asexual reproduction and aspects of the body plan required for preserving whole body regeneration. The link may mean their functions in body plan patterning underlie the differential retention and modification of regenerative abilities.
“We’ve never looked at this in these animals before,” he said. “Now, we’re finding new things that are either unique to these regenerative flatworms, shared amongst highly regenerative animals, or happening just below the surface in other animals too. We’re just starting to learn all the different ways these genes can create the diversity in the tree of life.”