The promise of human organotypic tissues for tissue development, disease modelling, and drug testing is widely recognized, but limitations around cell density, vascular structure, and scale must be solved before that potential is realized.
Credit: By Zhenzhen Zhou, Changru Liu, Yuting Guo, Yuan Pang and Wei Sun.
The promise of human organotypic tissues for tissue development, disease modelling, and drug testing is widely recognized, but limitations around cell density, vascular structure, and scale must be solved before that potential is realized.
Nearly all organotypic tissue engineering protocols involve the co-culture of parenchymal and endothelial cells, thereby generating vascularized material-free microtissues (high cell density) or bulk hydrogel tissues (low cell density). Several protocols take advantage of sacrificial 3D bioprinting to enhance cell density and scale, but these protocols also rely on large vessel channels to supply oxygen and nutrients, and lack a network of capillaries.
Module assembly (MoA) offers the ability to precisely control micro-niche patterning in three-dimensional space at cell density, multicellular composition, and tissue scale, representing a potential solution to these challenges.
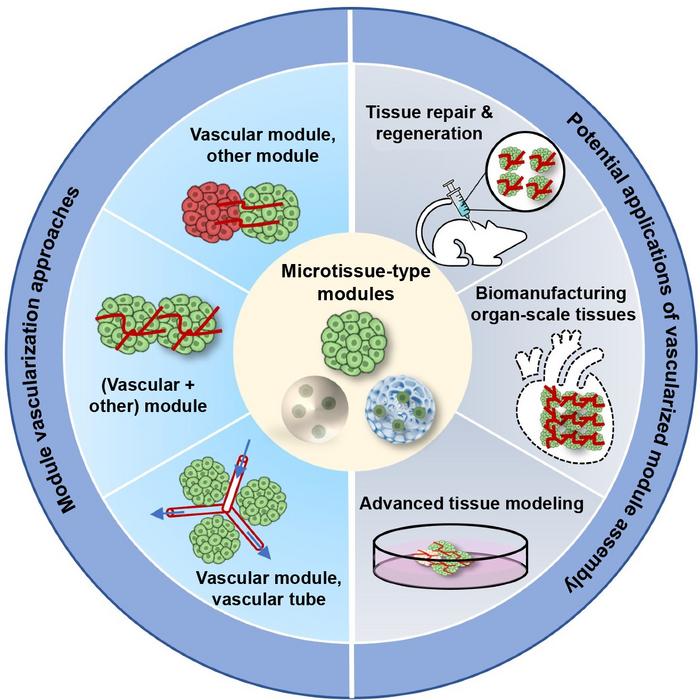
In the latest issue of International Journal of Extreme Manufacturing, Zhenzhen Zhou and co-workers present a review on engineering vascularized organotypic tissues that delivers the representative findings of different MoA approaches, and expansively discuss their effectiveness in tissue repair and regeneration, organ-scale tissue construction, and advanced tissue modelling.
The authors introduced module types for engineering organotypic constructs, including scaffold-free (spheroid and organoid) and scaffold-based (porous microsphere and microgel) microtissues.
These modules were then assembled through various methods, such as device-assisted culture, microfluidic chips and bioprinting. This method was able to rapidly and efficiently develop various organotypic tissues, such as multi-organ systems, organ-scale tissues, and complex tissue models, in contrast to the conventional cell-based assembly method.
A major limitation of the organotypic tissue field concerns the lack of functional vasculature. The authors summarized the pre-vascularization strategies for modules, including co-culturing parenchymal and endothelial cells before or after module preparation. Indeed, these findings reflect the importance of using pre-vascularized multicellular units for successful modelling in contrast to conventional single-cell units. Whether or not better tissue models can be generated by incorporating endothelial cells/progenitors before module preparation remains to be seen.
The validity of the MoA also allowed the use of the pre-vascularized modules for developing a well-organized vasculature, thus improving the efficacy of cell therapy in treating disease or disease-associated defects, such as heart, skeletal muscle, bone, diabetic complications, and peripheral artery disease.
One of the challenges with organ-scale tissue manufacturing is the lack or insufficiency of vascular networks. The pre-vascularized MoA approach offers a substantial improvement in constructing tissues with organotypic cellular density, microstructure, and function.
The authors also summarized the application of pre-vascularized MoA in producing advanced 3D models for the study of tissue development, pathogenesis, and drug screening. This approach demonstrates a great superiority in recapitulating the highly complex in vivo milieu and vasculature-mediated cell-cell/environment interactions.
In summary, the utilization of microtissues as basic modules represents an important step for the rapid generation of up-scaled functional tissue substitutes with a high cell density, well-organized microarchitecture, and complete vasculature. This review will no doubt catalyze further investigation into the modality in which MoA can be used to improve tissue engineering and regenerative medicine in tissue function, scale, and complexity. However, several issues remain to be addressed, such as standardization of pre-vascularization routes, improvement of vasculature quality, standardization of culture protocols, and match between assay methods and platforms.
About IJEM:
International Journal of Extreme Manufacturing (IF: 14.7, 1st in the Engineering, Manufacturing category in JCR 2023) is a new multidisciplinary, double-anonymous peer-reviewed and diamond open-access without article processing charge journal uniquely covering the full spectrum of extreme manufacturing. The journal is devoted to publishing original articles and reviews of the highest quality and impact in the areas related to the science and technology of manufacturing functional devices and systems with extreme dimensions (extremely large or small) and/or extreme functionalities, ranging from fundamental science to cutting-edge technologies that support the manufacturing of high-performance products involving emerging techniques and breaking the limits of currently known theories, methods, scales, environments, and performance.
Visit our webpage, Like us on Facebook, and follow us on Twitter and LinkedIn.
Journal
International Journal of Extreme Manufacturing
DOI
10.1088/2631-7990/acfcf2
Article Title
Engineering vascularized organotypic tissues via module assembly
Article Publication Date
11-Oct-2023



