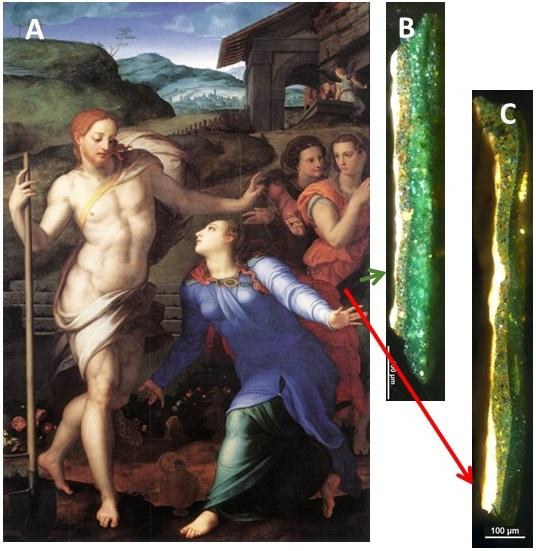
Credit: Adapted from Inorganic Chemistry 2019, DOI: 10.1021/acs.inorgchem.9b02007
Enticed by the brilliant green hues of copper acetate and copper resinate, some painters in the Renaissance period incorporated these pigments into their masterpieces. However, by the 18th century, most artists had abandoned the colors because of their tendency to darken with time. Now, researchers reporting in ACS’ journal Inorganic Chemistry have uncovered the chemistry behind the copper pigments’ color change.
Copper acetate (also known as verdigris) and copper resinate were used in European easel paintings between the 15th and 17th centuries. Artists typically mixed these pigments with linseed oil to make paint. Until now, scientists didn’t know why the green paints often turned brown with time, although they had some clues. Light exposure was thought to play a role because areas of paintings protected by frames remained green. Also, oxygen appeared to contribute to the darkening process, with the brown color spreading from cracks in the paint that exposed the underlying copper pigments to air. So Didier Gourier and colleagues wanted to analyze the chemical changes that occur in the paints upon light exposure.
The team determined that the molecular structures of copper acetate and copper resinate were quite similar: Both had two copper atoms bridged by four carboxylate groups, but there was more space between resinate than acetate molecules. The researchers mixed the pigments with linseed oil and spread them in a thin layer. They then exposed the paint films to 16 hours of 320-mW LED light, which corresponded to hundreds of years of museum light. This illumination caused bridging molecules between the pair of copper atoms to be lost, which were then replaced by an oxygen molecule, creating bimetallic copper molecules responsible for the brown color. This process occurred more readily for copper resinate than for copper acetate. Boiling the linseed oil before mixing, which some artists did to improve the drying process, slowed the darkening reaction.
###
The authors acknowledge funding from the French Foundation for Cultural Heritage Sciences and LabEx Patrima.
The abstract that accompanies this study is available here.
The American Chemical Society, the world’s largest scientific society, is a not-for-profit organization chartered by the U.S. Congress. ACS is a global leader in providing access to chemistry-related information and research through its multiple databases, peer-reviewed journals and scientific conferences. ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies. Its main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive news releases from the American Chemical Society, contact [email protected].
Follow us on Twitter | Facebook
Media Contact
Katie Cottingham
[email protected]
Related Journal Article
http://dx.




