The dynamic interplay between cellular motility and population growth within multicellular spheroids has recently emerged as a captivating frontier in biophysical research. In groundbreaking work spearheaded by scientists at the Max Planck Institute for Dynamics and Self-Organization (MPI-DS), a computational lens has been focused on how cells within growing three-dimensional colonies migrate and mix, revealing counterintuitive mechanical principles governing collective cellular behavior. This study uncovers a critical transition in cell mixing driven not solely by biochemical cues, but by an intrinsic mechanistic balance between motility and proliferation rates, offering profound implications for developmental biology, cancer research, and tissue engineering.
Cell motility is central to myriad biological processes — from embryogenesis and wound healing to immune response and cancer metastasis. Typically, cells crawl or push through their microenvironment, dynamically rearranging themselves to facilitate growth and adaptation. Yet, when cells proliferate rapidly to expand a colony or tissue, space constraints and mechanical forces emerge as dominant factors influencing whether cells can effectively migrate. The MPI-DS team reconstructed this tension in silico, developing a minimal but robust computational model simulating spheroidal cellular aggregates undergoing exponential growth while each cell was endowed with active motility forces.
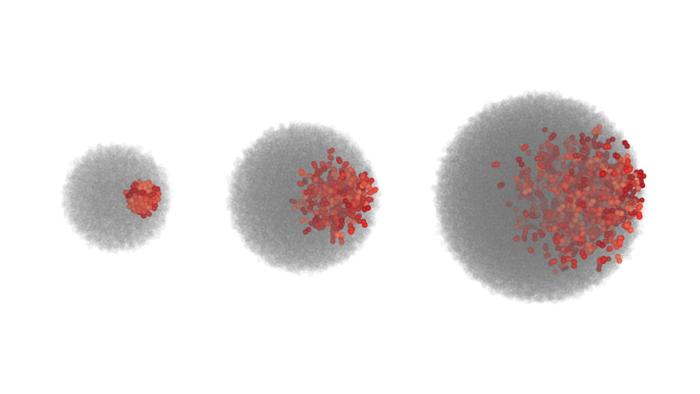
Their simulations yielded a striking discovery: increasing the growth rate — i.e., the frequency of cell divisions — paradoxically restricts the ability of cells to migrate within the colony, causing the system to transition from a highly mixed state to one where cells remain largely immobilized despite possessing motility machinery. This suppression of mixing occurs below a sharply defined threshold ratio of motility to growth rate, pinpointing a physical mechanism whereby unchecked proliferation frustrates cellular motion. Crucially, this finding defies the intuition that active movement would always overcome spatial limitations; instead, the emergent collective behavior resembles a phase transition governed purely by physical parameters.
Torben Sunkel, the first author, highlights that the observed motility inhibition is not an artifact of biochemical signaling adjustments but arises intrinsically from mechanical feedback present in crowded cellular environments. Cells embedded in densely packed tissues experience steric hindrance and mechanical jamming, impeding their migration paths. Furthermore, the radial expansion of the colony exponentially increases the effective distance a cell must traverse to reposition within the tissue. These dual constraints — mechanical crowding and geometric scaling — synergistically reduce the efficacy of cell-generated motile forces, thereby demarcating the sharp transition observed in the system.
Beyond theoretical implications, this research interfaces profoundly with experimental biology and medical sciences. Understanding the parameters that govern when cellular colonies transition between motile, mixed states and arrested, segregated arrangements can inform therapeutic strategies targeting tumor progression, where rapid proliferation and invasive motility co-occur. The revealed motility-growth threshold may serve as a diagnostic or prognostic biomarker, indicating when cancerous tissues become mechanically constrained or poised for metastasis.
Moreover, the principles elucidated extend to bacterial biofilms, where spatial organization dictates resilience and antibiotic susceptibility, as well as to wound healing, where orchestrated cell migration is essential for tissue repair. Tissue engineering — an arena seeking to construct functional artificial tissues — could benefit from manipulating proliferation and motility parameters to optimize scaffold colonization and cellular intermixing, thus recapitulating native tissue architectures with enhanced fidelity.
Significantly, the computational framework introduced allows for precise tuning of motile force amplitudes and division rates, enabling systematic exploration of parameter spaces inaccessible in vitro. Such control paves the way for predictive modeling of complex multicellular systems, accelerating both fundamental insight and translational applications. The minimalist approach further underscores that even simplified representations, when grounded in realistic physics, capture essential biological phenomena missed by overly complex models.
The concept of a “motility-induced mixing transition” propels forward our understanding of growth-driven mechanical regulation within multicellular structures. Its identification as a sharp, threshold-dependent process provides a mechanistic basis for the spatial heterogeneity observed in expanding tissues and tumors. Intriguingly, it suggests potential evolutionary pressures to optimize the balance of motility and proliferation for tissue functionality or pathological progression, a theme ripe for future empirical investigation.
From a broader physics standpoint, this work bridges cellular biology with nonequilibrium statistical mechanics, highlighting how biological systems naturally organize through transitions reminiscent of jamming and glassy dynamics. The observed phenomena bear resemblance to phase behaviors in active matter systems, wherein individual units’ intrinsic activity and interactions govern emergent collective states. By situating living tissues within this framework, the study opens avenues for interdisciplinary collaborations leveraging physics to unravel biological complexity.
Encouragingly, the visualization of migrating cells in the growing colonies — vividly displaying extensive mixing under suitable motility-to-growth ratios versus sharply diminished movement otherwise — promises to inspire in vivo or in vitro experiments aimed at validating and extending these predictions. Fluorescence lineage tracing or live-imaging techniques in multicellular spheroids could directly test the sharpness of this transition and probe underlying molecular mechanisms modulating motility forces.
Overall, this study exemplifies how combining computational modeling with fundamental physics reveals surprising biological truths, challenging conventional expectations. By clarifying how rapid growth can paradoxically immobilize inherently motile cells through purely mechanical effects, it reshapes our conceptual frameworks governing development, disease, and regeneration. This rich interface between physics and biology is poised to yield further insights revolutionizing how we interpret and manipulate living matter.
Subject of Research: Motility and growth interactions in multicellular spheroids, cellular migration dynamics, collective cell behavior
Article Title: Motility-induced mixing transition in exponentially growing multicellular spheroids
News Publication Date: 24-Apr-2025
Web References: https://doi.org/10.1038/s42005-025-02090-5
Image Credits: MPI-DS, LMP
Keywords
Cell migration, Bacterial growth, Tumor growth, Cell growth, Motion
Tags: active motility forces in cellsbiophysical research breakthroughscell colonies growth dynamicscell proliferation and space constraintscellular motility and migrationcomputational modeling in biologydevelopmental biology studiesimplications for cancer researchintrinsic mechanistic balance in cellsmechanical principles of cell behaviormulticellular spheroids researchtissue engineering advancements