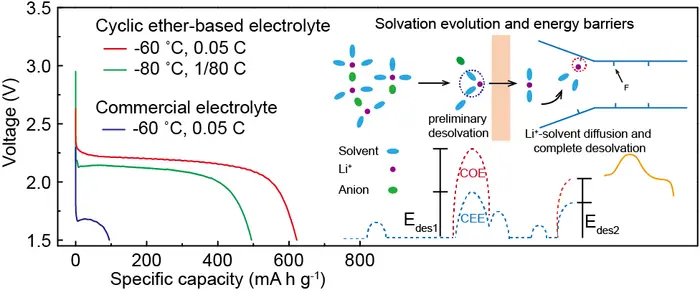
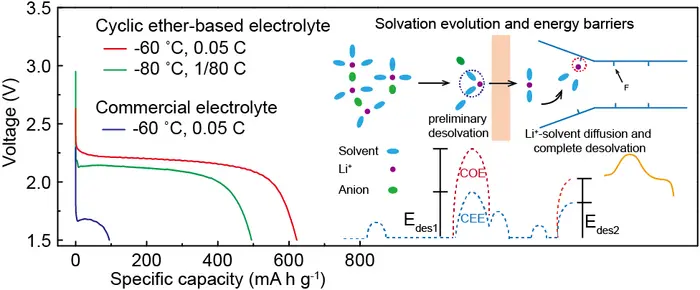
By selecting low melting point solvents and suitable salts and precisely tailoring Li+-coordinated environment via integration of low-affinity solvents with moderate anions, the weakly solvating cyclic ether-based electrolyte is introduced to significantly decrease desolvation activation energies and facilitate reaction kinetics, which are proved by Molecular dynamics and binding energy calculation .
Credit: ©Science China Press
By selecting low melting point solvents and suitable salts and precisely tailoring Li+-coordinated environment via integration of low-affinity solvents with moderate anions, the weakly solvating cyclic ether-based electrolyte is introduced to significantly decrease desolvation activation energies and facilitate reaction kinetics, which are proved by Molecular dynamics and binding energy calculation .
Discharged until 1.5V cut-off voltage, the assembled CFx/Li batteries yield 723, 652, and 495 mA h g-1 provided with average output voltages of 2.26, 2.22, and 2.11 V at -40, -60, and -80 °C, corresponding to energy densities of 1634, 1447 and 1044 W h kg-1, the best performances among current organic liquid electrolyte to the best of our knowledge. The higher capacity retention up to 82 % is produced at a discharge rate of 15 C (25°C). To further testify potential for practical applications, the assembled CFx/Li cells with high load increased to 18-22 mg cm-2 still show energy densities of 1683 and 1395 W h kg-1 at -40 and -60˚C
Researchers propose powerful strategy for electrolyte modification with cost-effectiveness and practical scalability, which has unexploited application value and favorable exploitation foreground for ultralow-temperature primary battery
See the article:
Weakly-Solvating Electrolyte Enables Ultralow Temperature (-80 ℃) and High-Power CFx/Li Primary Batteries
https://doi.org/10.1007/s11426-023-1638-0
Journal
Science China Chemistry
DOI
10.1007/s11426-023-1638-0