A groundbreaking study from Hiroshima University has unveiled the intricate developmental choreography behind the formation of the musculoskeletal system. By harnessing advanced genetic engineering techniques and state-of-the-art fluorescence imaging, the research team has illuminated the dynamic interactions that occur between cartilage, tendons, and ligaments during embryogenesis. This innovative approach not only overcomes the limitations imposed by traditional histological methods but also opens a new window into understanding how the structural components of the musculoskeletal system seamlessly integrate to support movement, stability, and bodily function.
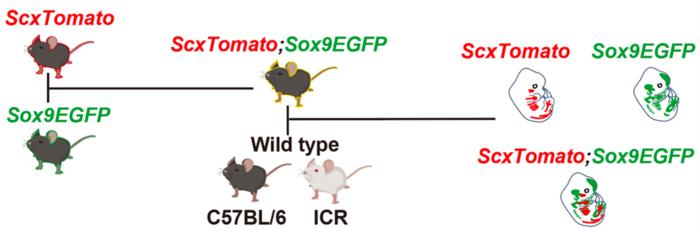
At the heart of this investigation lies a novel double-reporter mouse model, engineered to simultaneously visualize two critical molecular markers involved in the differentiation and spatial organization of musculoskeletal tissues. The model employs the red fluorescent protein driven by the Scleraxis (Scx) gene promoter, marking sites of tendon and ligament emergence, while green fluorescent protein expression is driven by the Sox9 gene, delineating chondrogenic territories. By crossing ScxTomato transgenic mice with Sox9EGFP knock-in mice, the authors created a powerful tool allowing live visualization of the developing communication and connectivity between these tissues in a three-dimensional embryonic landscape.
This study exploits cutting-edge tissue clearing techniques that render embryonic structures optically transparent without disrupting cellular morphology or protein expression. When combined with high-resolution fluorescence microscopy, this approach enables detailed imaging of the spatial relationships and temporal dynamics between tendinous and cartilaginous primordia. Such technology surpasses traditional two-dimensional sectioning methods, which inevitably lose critical information regarding the complex 3D arrangements essential for functional musculoskeletal integration.
Scleraxis (Scx) is a transcription factor widely recognized as a master regulator of tendon and ligament development. Its expression specifically marks progenitor cells that will give rise to these connective tissues. On the other hand, Sox9, another pivotal transcription factor, governs cartilage formation by regulating chondrocyte differentiation and proliferation. The novel double-fluorescent reporter model uniquely enables simultaneous observation of these two molecular landscapes, revealing the nuances of how tendon and cartilage primordia coordinate their development in time and space.
The experimental findings reveal that tendons and ligaments, marked by red fluorescence, navigate alongside green fluorescent cartilage structures during organogenesis. This spatial juxtaposition underlies the precise connectivity necessary for muscle attachment and skeletal articulation. The researchers observed distinct populations co-expressing Scx and Sox9 to varying degrees, suggesting a heterogeneous cell population that could represent transient states in differentiation or specialized progenitor niches contributing to tissue integration.
Further elucidating the role of Scx, the research team generated Scx-deficient mice and demonstrated that loss of this factor disrupts not only tendon and ligament maturation but also alters muscle morphology and its anchoring to cartilaginous bone primordia. These insights underscore Scx’s broader influence in shaping the musculoskeletal framework beyond its traditionally understood scope, revealing new layers of regulatory complexity in tissue development.
The significance of Scx and Sox9 co-expression heterogeneity might extend beyond development into processes of tendon and ligament regeneration and degeneration. Variability in expression levels within the Scx^+/Sox9^+ cellular cohort could underpin different functional states or lineage trajectories, offering avenues for understanding musculoskeletal disorders and age-related tissue deterioration.
This pioneering research has broad implications for regenerative medicine and orthopedics. Decoding the developmental cues governing tendon-cartilage-muscle integration may facilitate the design of biomimetic scaffolds and targeted therapies aimed at repairing or regenerating damaged connective tissues resulting from trauma or degenerative diseases.
Looking forward, investigators aim to combine the double-reporter system with other genetically engineered mouse lines to dissect the molecular mechanisms orchestrating musculoskeletal assembly in even finer detail. Moreover, extending these analyses to postnatal and adult stages using advanced tissue clearing and 3D imaging technologies could shed light on how tendons and ligaments mature, adapt, and respond to injury throughout life.
This interdisciplinary effort, involving experts from multiple prestigious institutions, is a testament to the power of genetic innovation and imaging technology in revealing the unseen intricacies of organogenesis. By bridging molecular genetics, developmental biology, and biomedical imaging, the study paves the way for transformative progress in understanding the architecture and function of the musculoskeletal system.
In summary, the use of the ScxTomato;Sox9EGFP double-reporter mouse model combined with high-resolution fluorescence imaging marks a significant leap in visualizing and interpreting the formation of the musculoskeletal system. This strategy provides an unprecedented dynamic 3D perspective on how tendinous and cartilaginous tissues interact, mature, and integrate with musculature to form a cohesive biomechanical entity capable of supporting life’s essential functions.
The results, published in the March 2025 issue of the journal Development, underline the critical contributions of transcription factors Scleraxis and Sox9 in governing the spatial and temporal ordering needed for proper musculoskeletal assembly. This research unravels the sophisticated developmental symphony directing the formation of connective tissues and offers promising new directions for tackling musculoskeletal pathologies.
Subject of Research: Musculoskeletal system development and integration; fluorescent reporter mouse models for tendon, ligament, and cartilage visualization.
Article Title: Dynamic interactions between cartilaginous and tendinous/ligamentous primordia during musculoskeletal integration
News Publication Date: 26-Mar-2025
Web References:
Development DOI: 10.1242/dev.204512
References: Yu et al., Development, 2025
Image Credits: Reproduced with permission from Development, Yu et al., 2025.
Keywords: Musculoskeletal system, Developmental biology, Genetics, Anatomy, Tendon formation, Cartilage development, Scleraxis (Scx), Sox9, Fluorescent imaging, Organogenesis
Tags: 3D visualization techniquescartilage and tendon interactionsdouble-reporter mouse modelembryonic tissue organizationfluorescence imaging in researchgenetic engineering in embryologyinnovative approaches in developmental biologyintegration of musculoskeletal componentslive visualization of tissue developmentmusculoskeletal system developmentScleraxis and Sox9 gene rolestissue clearing methods in biology