An artificial pancreas developed at the University of Virginia Center for Diabetes Technology improves blood sugar control for people ages 2 to 72 with type 1 diabetes, according to a new combined analysis of three clinical trials.
Credit: Tandem Diabetes Care
An artificial pancreas developed at the University of Virginia Center for Diabetes Technology improves blood sugar control for people ages 2 to 72 with type 1 diabetes, according to a new combined analysis of three clinical trials.
Across the three trials, participants using the artificial pancreas spent an average of 2.8 more hours per dayin their target blood sugar range compared with participants in control groups who used standard methods for managing their blood sugar.
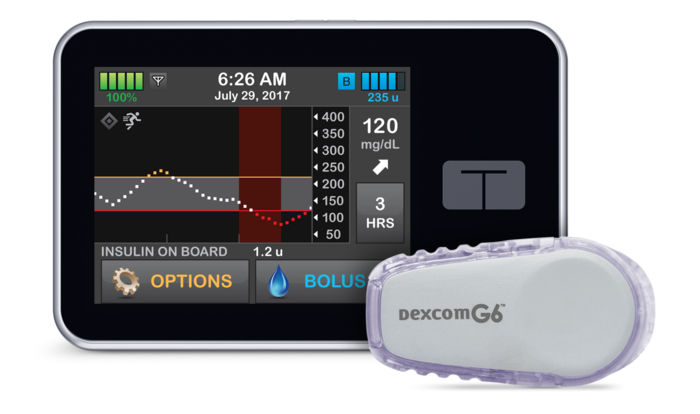
Manufactured by Tandem Diabetes Care and sold as the Control-IQ system, the artificial pancreas is a diabetes-management solution that automatically monitors and regulates blood glucose. The device has an insulin pump that uses advanced control algorithms based on the person’s glucose-monitoring information to adjust the insulin dose as needed. The U.S. Food and Drug Administration has approved the system for people ages 6 and older with type 1 diabetes.
Consistent Improvements
The new analysis was based on findings from 369 participants ages 2 to 72 who joined trials at eight U.S. sites, including UVA Health. Among those participants, 256 were assigned to use the artificial pancreas system, with the remaining 113 assigned to a control group.
On average, the time participants using the artificial pancreas spent within their target blood glucose range was 13 percentage points higher than for participants in the control group. Benefits were seen both during the day and at night, though there was greater improvement overnight. Participants using the artificial pancreas spent significantly more time within their target blood sugar range within one day of beginning to use the system, the analysis showed.
The artificial pancreas also significantly decreased participants’ hemoglobin A1c (average blood sugar) levels. The average hemoglobin A1c levels of system users dropped from 7.5% to 7%, compared with a decrease from 7.6% to 7.5% in the control groups.
The improvements in both hemoglobin A1c levels and time spent within the target blood sugar range were consistent across all ages, the researchers found, as well as across racial and ethnic groups and regardless of household income level and how participants had previously managed their diabetes.
“All subgroups in these studies, regardless of age, ethnicity, education or previous pump experience, benefitted from Control-IQ technology,” said Boris Kovatchev, PhD, director of the UVA Center for Diabetes Technology. “It is clear from these results, which are consistent with real-life data from thousands of current Control-IQ technology users, that this technology should be strongly considered as an option for anyone living with type 1 diabetes.”
Findings Published
The study results have been published in the journal Diabetes Technology & Therapeutics. The study’s authors are Roy W. Beck, Lauren G. Kanapka, Marc D. Breton, Sue A. Brown, R. Paul Wadwa, Bruce A. Buckingham, Craig Kollman, and Kovatchev.
The three studies included in the review were funded by the National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases, grants UC4 108483 and U01DK127551. The findings from the studies were originally summarized in three papers published in the New England Journal of Medicine. Kovatchev served as principal investigator of grant UC4108483, as did UVA’s Breton on U01DK127551. Tandem Diabetes Care provided the investigational closed-loop systems used in the trials, while Dexcom Inc. provided the continuous glucose monitors.
Journal
Diabetes Technology & Therapeutics



