A groundbreaking discovery by researchers at POSTECH has shed light on the underwater adhesion mechanism of hairy mussels, specifically the species Barbatia virescens. This study, spearheaded by Professor Dong Soo Hwang and Research Professor Jimin Choi, highlights an innovative approach to understanding how these marine creatures adhere to surfaces even in challenging wet environments. Published in the renowned journal Nature Communications, their research uncovers crucial insights into the molecular interactions that govern these natural adhesive mechanisms.
For decades, scientists have observed the remarkable ability of marine organisms, such as mussels and barnacles, to maintain strong adhesive bonds in extremely wet conditions. Yet, despite extensive research, the molecular underpinnings of this adhesion, particularly those involving the epidermal growth factor (EGF) domain, remained somewhat of a mystery. The EGF domain had been identified as a fundamental component of mussel adhesive proteins nearly 40 years ago, but the exact mechanics of how these proteins worked in underwater conditions sparked significant intrigue within the scientific community.
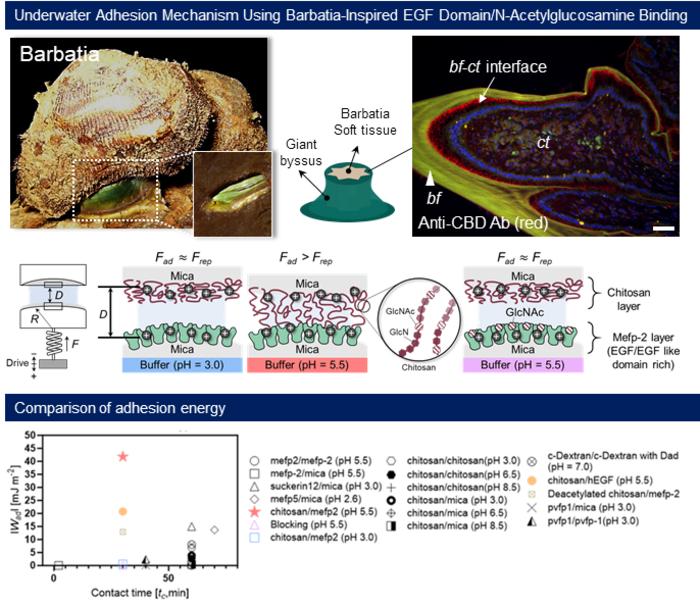
The researchers conducted rigorous experimental tests to quantify the adhesion energy presented by these specific protein interactions. Impressively, they discovered that the adhesion energy produced by the EGF-GlcNAc interaction was more than three times that of some of the most recognized wet-adhesive proteins, such as the mussel foot protein (mefp-5) and the spider silk protein known as suckerin. This substantial difference in adhesion energy suggests that the mechanisms driving this type of adhesion are not just more efficient but potentially transformative for various applications.
One of the most profound insights emerging from this research involves the revelation that the adhesion process does not rely on oxidation, a feature that has long characterized traditional DOPA-based adhesives. For many years, the activities of these natural adhesives were primarily attributed to the oxidation of particular amino acids in the adhesive proteins. The POSTECH team’s discovery of an oxidation-independent adhesion mechanism conveys potential new pathways for developing innovative and versatile adhesives across multiple fields.
Additionally, the implications of these findings extend far beyond basic biological understanding. Research Professor Jimin Choi highlighted the significance of GlcNAc, which is not only prevalent in many biological tissues but is also found in biofilms. This raises exciting prospects for developing adhesives that find application in bioelectronics, tissue engineering, and environmentally friendly antifouling coatings, among other related fields.
In advancing their research agenda, Professor Dong Soo Hwang emphasized the importance of these findings in innovating sustainable and high-performance adhesives for both underwater applications and medical-grade bioadhesives. Moreover, this research is seen as a critical stepping stone toward broader applications in the adhesive industry, promoting an intersection of materials science and biological research in the development of solutions that are effective, reversible, and applicable in varied environments.
The findings articulated by the POSTECH researchers stem from meticulous funding support provided by the National Research Foundation of Korea (NRF). This backing has been pivotal, allowing for extensive theoretical and practical research under the NRF’s 2022 Basic Research Program, directed by the Ministry of Science and ICT alongside the Ministry of Education. Such institutional support underscores the significance of this research, laying the groundwork for potential future advancements in adhesive technology emanating from biological principles.
As the scientific community begins to digest these findings, the relevance of EGF and GlcNAc in creating powerful adhesive solutions can now be re-examined in both natural and synthetic contexts. The revisitation of biological adhesives through a modern lens could inspire advancements not only in adhesive development but significantly integrate these discoveries into the realms of bioengineering and biotechnology. Overall, the POSTECH team’s revelations mark a noteworthy chapter in the ongoing exploration of marine biology and materials science.
This research not only enhances our comprehension of natural adhesion but also nudges the very formulation of adhesives for practical applications. The seamless blending of natural mechanisms with technological advancements opens a new frontier filled with possibilities, waiting to be explored by both scientists and industries. From sustainable solutions to applications that could span diverse fields, the implications of their findings promise a fascinating trajectory into the future of adhesive technology.
Furthermore, the integration of engineering with biology could overwrite conventional paradigms, rendering the ethos of materials science more fluid and adaptive than ever before. This dynamism, propelled by investigations fanatical in detail and broad in scope, emphasizes a pivotal transition phase where interdisciplinary studies transform raw biological phenomena into functional applications. The POSTECH research team should be commended for illuminating this vital area of molecular biology and materials science, providing a beacon of progress as we look toward an era of advanced bioadhesive innovation.
The journey of unraveling the complexities of natural adhesion mechanisms has only just begun. With every discovery, researchers glean insights that not only enrich academic understanding but also enhance industry capabilities, ultimately fostering a more profound connection between biology and technology. Through continued inquiry and innovation, the potential applications of mechanically optimized biological adhesives will invariably reinvigorate many fields, bridging gaps that once seemed insurmountable. Scholars and practitioners alike eagerly anticipate what lies ahead in this evolving narrative.
Subject of Research: Underwater Adhesion Mechanism of Hairy Mussels
Article Title: Sticky organisms create underwater biological adhesives driven by interactions between EGF- and GlcNAc- containing polysaccharides
News Publication Date: [Not Provided]
Web References: [Not Provided]
References: [Not Provided]
Image Credits: POSTECH
Keywords: EGF, GlcNAc, underwater adhesion, mussels, biological adhesives, molecular mechanisms, sustainable technology, POSTECH, Nature Communications.
Tags: Okay