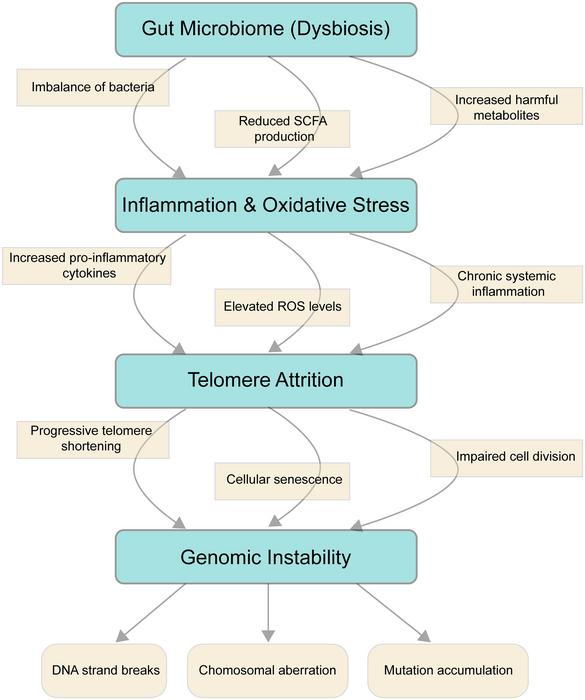
Aging remains one of the most complex biological phenomena, governed by a mosaic of interdependent molecular and cellular processes. Among these, genomic instability and telomere attrition are universally recognized as central hallmarks that drive progressive physiological decline. Recent advances in microbiome research have uncovered a profound connection between the gut microbial ecosystem and the integrity of our genome, unveiling a novel axis influencing the aging trajectory. This emerging paradigm positions the gut microbiota not merely as a passive factor but as an active player modulating genomic health and telomeric maintenance, with wide-reaching implications for age-associated diseases and longevity.
The human gut microbiota comprises trillions of microorganisms forming a highly dynamic community that fluctuates in composition and function throughout life. Dysbiosis, the perturbation of this delicate balance between beneficial and pathogenic microbes, initiates cascades of systemic inflammation and oxidative stress that exacerbate DNA damage. Reactive oxygen species (ROS) generated during this state accelerate telomere erosion by overwhelming cellular antioxidant defenses and impairing DNA repair pathways. At the molecular level, the microbiota exerts influence through metabolites such as short-chain fatty acids (SCFAs), which serve as crucial modulators of inflammation and genome stability. Reduced SCFA levels, frequently observed in aged or dysbiotic guts, correlate with diminished telomerase activity, thereby hastening telomere shortening.
Crucially, microbial endotoxins and genotoxins represent direct molecular insults to genomic material. Certain bacteria—including Helicobacter pylori and Fusobacterium nucleatum—produce compounds that either generate ROS or directly interfere with DNA repair enzymes, contributing to chromosomal aberrations. Furthermore, commensal strains like Escherichia coli and Bacteroides fragilis have been identified as sources of genotoxins such as colibactin, molecules known to induce double-stranded DNA breaks. These interactions create an environment ripe for mutagenesis and genomic instability, phenomena that underlie major age-related pathologies including carcinogenesis and neurodegeneration.
The amplification of chronic, low-grade inflammation—commonly termed “inflammaging”—further compounds genomic insults. Pro-inflammatory cytokines released in response to microbial imbalance can suppress nucleotide excision repair and homologous recombination, two key DNA repair mechanisms. This impairment results in accumulation of DNA lesions that compromise chromosomal integrity. Intriguingly, therapeutic interventions such as fecal microbiota transplantation (FMT) and antibiotic modulation have shown promise in restoring microbial homeostasis, lowering systemic inflammatory markers, and consequently enhancing DNA repair efficacy in preclinical aging models.
Telomere biology, pivotal in cellular longevity, is tightly regulated by shelterin complexes that protect chromosome ends. Aging-associated dysbiosis disrupts this regulation by fostering oxidative environments that accelerate telomeric DNA damage. Studies reveal that individuals with reduced microbial diversity tend to possess shorter telomeres, while populations exhibiting exceptional longevity, like centenarians, maintain gut communities enriched in anti-inflammatory and telomere-preserving taxa such as Akkermansia muciniphila and Bifidobacterium species. These microbes promote SCFA production, which in turn upregulates telomerase reverse transcriptase, safeguarding telomere length and delaying cellular senescence.
The gut microbiome’s influence extends beyond local effects, interfacing with systemic metabolic networks and immune signaling pathways. Metabolites derived from bacterial fermentation modulate epigenetic programming and mitochondrial dynamics, both of which intersect intricately with genomic stability. For example, SCFAs act as histone deacetylase inhibitors, modifying chromatin architecture to support genome maintenance. Moreover, a balanced microbial ecosystem curtails the accumulation of toxic secondary bile acids like deoxycholic acid, known to induce DNA strand breaks and propagate oncogenic transformation.
Research into centenarian microbiomes offers compelling insights into microbially mediated mechanisms of healthspan extension. Okinawan and Sardinian populations renowned for longevity exhibit gut microbial signatures that bolster mucosal barrier integrity, reduce systemic oxidative stress, and attenuate inflammatory pathways implicated in telomere attrition. These microbial profiles correlate with enhanced mitochondrial function and sustained genomic fidelity, epitomizing a symbiotic relationship that promotes resilience against age-related degeneration.
While causality remains to be definitively established, emerging clinical trials employing microbiome-targeted therapies aim to harness these protective mechanisms. Anti-inflammatory drugs such as canakinumab and metabolic modulators like metformin demonstrate the potential to mitigate DNA damage accumulation and preserve telomeric integrity in aged individuals. Concurrently, FMT and probiotic interventions enrich beneficial microbial populations, restoring SCFA synthesis and reducing deleterious metabolites. These strategies signify a paradigm shift toward integrated approaches that leverage the microbiome’s regulatory capacity to counteract biological aging.
Future research agendas emphasize a “meta-hallmark” concept, recognizing the interconnectedness of microbiome dynamics with systemic aging pathways. Deciphering the complex crosstalk between gut microbes, host immune responses, and genome maintenance machinery at single-cell resolution promises to uncover novel biomarkers and therapeutic targets. Personalized microbiome modulation, guided by high-throughput sequencing and metabolomic profiling, holds potential to revolutionize interventions aimed at extending healthspan by preserving genomic integrity.
In conclusion, the microbiome emerges as a master regulator at the nexus of aging biology, intricately entwined with the mechanisms governing genomic stability and telomere dynamics. Its dualistic nature—capable of either accelerating or decelerating aging processes depending on community composition and function—underscores the necessity of maintaining microbial homeostasis for healthy longevity. As researchers continue to unravel this hidden driver of aging, the prospect of mitigating senescence and age-associated diseases through microbiome-based therapies becomes increasingly tangible, charting a new frontier in biogerontology and precision medicine.
Subject of Research: The influence of gut microbiota on genomic stability and telomere dynamics in aging.
Article Title: The Hidden Drivers of Aging: Microbial Influence on Genomic Stability and Telomere Dynamics
News Publication Date: 17-Apr-2025
Web References:
Exploratory Research and Hypothesis in Medicine journal: https://www.xiahepublishing.com/journal/erhm
DOI link: http://dx.doi.org/10.14218/ERHM.2024.00045
Image Credits: Swarup K. Chakrabarti, Dhrubajyoti Chattopadhyay
Keywords: Microorganisms, Gut microbiota, Genomic instability, Inflammation, Oxidative stress
Tags: age-associated diseases and longevitydysbiosis and systemic inflammationgenomic instability and telomere attritiongut health and cellular aginggut microbiota and agingimplications of microbiome research on agingmicrobial metabolites and inflammationmicrobiome influence on telomere maintenanceOxidative stress and DNA damagerole of microbiome in genome healthshort-chain fatty acids and agingtelomere erosion mechanisms