Credit: UNC Lineberger
CHAPEL HILL – University of North Carolina Lineberger Comprehensive Cancer Center researchers have achieved a major milestone with the launch of two clinical trials testing an experimental therapy in which patients' own immune cells are genetically engineered to fight their cancer.
The early-stage cellular immunotherapy trials are for patients with either Hodgkin lymphoma or non-Hodgkin lymphoma, who lack other treatment options or are at high risk of their disease returning. Researchers are working to open trials for other cancers.
"Remarkable advances have been made in how we treat cancer in the past decade, but there are still many cancers, including advanced lymphoma, for which there are few effective treatment options," said UNC Lineberger Director Norman E. Sharpless, MD. "Cellular immunotherapies hold tremendous promise, and the studies we are conducting today can put us in a position to offer more effective cancer treatments in the future."
UNC Lineberger's cellular immunotherapy program has come to fruition in a relatively short period of time. Center leaders recruited Gianpietro Dotti, MD, and Barbara Savoldo, MD, PhD, to UNC to lead the program in 2015. Last year, the center completed construction of a U.S. Food and Drug Administration-approved Good Manufacturing Practices (GMP), or "clean," facility in which to engineer immune cell-based therapies. With the opening of this facility, UNC Lineberger has become one of only a select academic centers in the United States with the capability to genetically modify patient immune cells for clinical use.
Dotti and Savoldo's recruitment, as well as critical support of the research initiative's infrastructure, were made possible by the University Cancer Research Fund, a landmark investment from the state of North Carolina for cancer research. Alice Lehman, the McMichael Family Foundation and the Barnhill Family Foundation also made significant philanthropic gifts to advance UNC Lineberger's cellular immunology program.
"The establishment of our cellular immunotherapy program is significant for several reasons," said Jonathan Serody, MD, associate director of translational science at UNC Lineberger and a medical oncologist in the UNC Lineberger Leukemia and Lymphoma Program. "First, it provides a dedicated center to rigorously investigate these experimental therapies. Second, it means people who live in southeastern U.S. can stay closer to home to undergo cellular immunotherapy treatment."
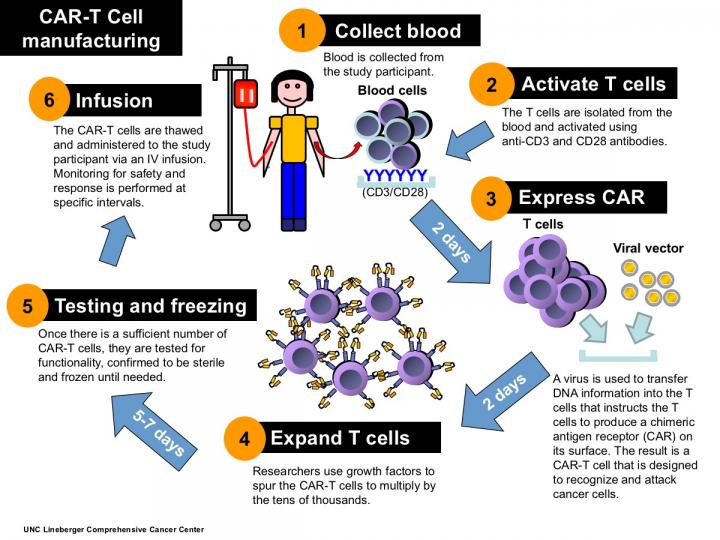
Cellular immunotherapy involves extracting disease-fighting immune cells – called T-cells – from the patient's blood, and genetically engineering them to recognize the patient's cancer. The researchers use a modified virus to insert DNA into the T-cells, which spurs the T-cells to express a receptor that allows them to recognize and destroy cancer cells. The hybrid T-cells, which are called chimeric antigen receptor T-cells, or CAR-T cells, are then multiplied by the tens of thousands and infused back into the patient. UNC Lineberger's first two clinical trials use T-cells engineered to recognize tumors expressing the CD30 protein marker.
UNC Lineberger's first trial is open for patients with relapsed or refractory Hodgkin or anaplastic non-Hodgkin lymphoma whose tumors express the CD30 antigen on the cell surface. Study participants will receive high-dose chemotherapy followed by an autologous stem cell transplant and infusion of the CAR T-cells directed against this CD30 target. The primary objective of the phase I trial is to gauge the safety of the treatment after transplant.
A second, phase Ib/II trial is open for patients 18 years and older with relapsed or refractory Hodgkin lymphoma and anaplastic non-Hodgkin lymphoma who are not candidates for autologous stem cell transplantation. The study is designed to assess the treatment's safety, as well as to estimate the response of the cancer and the patient's survival after treatment. Both trials require patients' tumors to be positive for the CD30 cell surface marker.
"While many patients with lymphoma often have excellent responses to currently available treatments, there are still a large number of them whose disease either does not respond to the initial treatment, or it relapses," said Thomas Shea, MD, UNC Lineberger member and medical director at the UNC Bone Marrow and Stem Cell Transplant Program. Shea is the principal investigator for both trials. "Although early in the process, we have reason to believe that this treatment approach will benefit a number of these relapsed patients just as has been shown for other diseases such as acute leukemia."
As the investigational treatments have proven safe, the clinical trials have progressed into gradually higher dose levels.
"We've clearly shown that the first dose levels, which are the lowest dose levels, are safe," Serody said. "We can grow the cells, we can give the cells, and we have seen limited toxicity so far with giving the cells. The next goal is to get the dose levels to the stage that may be associated with efficacy."
Additional trials are in development for patients with acute lymphoblastic leukemia, and whose tumors have the CD19 surface marker. The researchers also are planning trials for multiple myeloma as well as for certain brain cancers.
"We are investigating this promising treatment approach to address areas of critical need for patients who, right now, have few therapeutic choices to try to extend their lives," Serody said.
###
Media Contact
Bill Schaller
[email protected]
919-692-3405
http://cancer.med.unc.edu/
############
Story Source: Materials provided by Scienmag