Despite high hopes, a drug that wipes out the namesake cell type associated with the disease eosinophilic esophagitis (EoE) doesn’t make patients feel better and doesn’t reverse tissue damage in their throats.
Credit: Cincinnati Children’s
Despite high hopes, a drug that wipes out the namesake cell type associated with the disease eosinophilic esophagitis (EoE) doesn’t make patients feel better and doesn’t reverse tissue damage in their throats.
Meanwhile, data show that a different drug that had previously been approved for use in adults and teens with EoE is also safe and effective for children under 12 who weigh at least 15 kg (about 33 pounds).
The results of these clinical trials—plus an accompanying editorial—appear in the June 17, 2024, edition of The New England Journal of Medicine.
“Together, these trials provide exciting advances in our understanding of, and treatment options for, this increasingly common and perplexing disease,” writes Benjamin Wright, MD, an allergist-immunologist with The Mayo Clinic in Phoenix, AZ.
Long-time EoE expert Marc Rothenberg, MD, PhD, and colleagues at Cincinnati Children’s were deeply involved in both studies.
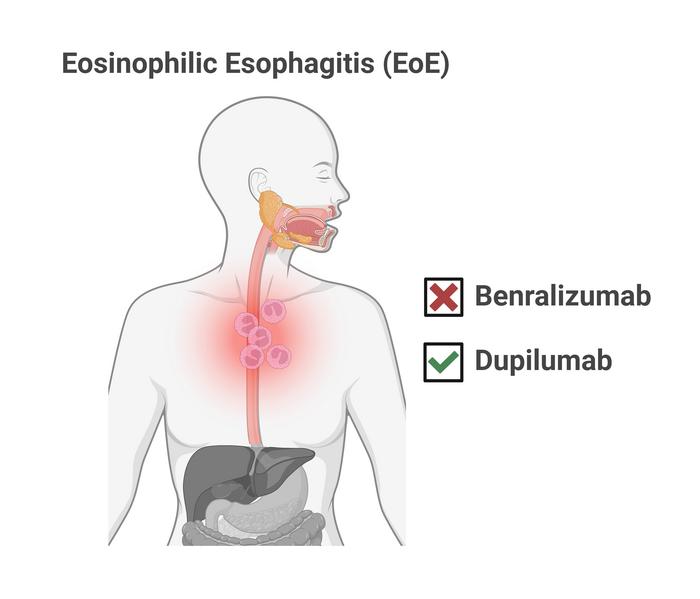
What is EoE?
This severe form of food allergy occurs in an estimated 180,000 people in the United States, including at least 21,000 children under 11. For those affected, a variety of foods can trigger a powerful immune response. Patients can experience painful throat inflammation, tissue damage, and difficulty swallowing. When not controlled, the ongoing disruption to a healthy diet can limit a child’s growth and lead to other complications.
The most distinguishing feature of EoE has been a well-documented spike in the number of eosinophils – a type of white blood cell – found in the throat. For many years this surge of immune cells also was believed to be the primary cause of the disease.
New findings from the Phase 3 MESSINA clinical trial add to a body of evidence suggesting otherwise.
Effective but not helpful
This study evaluated a monoclonal antibody treatment called benralizumab, which has been shown in other studies to be highly effective at reducing unwanted eosinophils. Somewhat like an exploding paint capsule making it easier for cops to catch a bank robber, the antibody binds to eosinophil cells and exposes them to patrolling natural killer cells.
In fact, this treatment has been approved for treating poorly controlled asthma because it helps break a feedback cycle of eosinophil-mediated hyperreaction and mucus production in inflamed airways. That asthma success inspired many scientists to hope that benralizumab would become a difference-maker in treating EoE.
But when tested in 211 patients with EoE—ages 12 to 65—people still experienced inflammation, difficulty swallowing, pain, and reduced quality of life. This occurred despite more than 80% of those taking benralizumab showing “near-complete depletion” of eosinophils in their blood and esophagus. Further, microscopic exams of tissue samples showed no significant improvement at a biological level.
“This trial calls into question the clinical relevance of monitoring EoE for treatment effect solely on the basis of the degree of eosinophilic inflammation,” says Rothenberg. “The MESSINA study highlights the importance of research as it was a very reasonable assumption that eosinophilic esophagitis would be caused by eosinophils. Based on these new results, we now know that this assumption is primarily incorrect.”
Rothenberg, who directs the Division of Allergy and Immunology at Cincinnati Children’s, served as corresponding author for the study. Evan Dellon, MD, MPH, with the University of North Carolina at Chapel Hill, was co-first author.
Extended approval for dupilumab
Benralizumab targets the activity of interleukin-5 (one of several proteins involved in our bodies’ immune response). But other research has shown better results against EoE by targeting two other interleukins at the same time: IL-4 and IL-13.
The US Food and Drug Administration initially approved the drug dupilumab for treating EoE in 2022, but only for adults and teens. Based on data from a more recent clinical trial focusing on younger children, the FDA acted in January 2024 to extend approval to children aged 1 to 12 who weigh at least 15 kg (about 33 pounds).
The data and related findings that supported that decision also were published in NEJM on June 27.
To date, dupilumab is the only FDA-approved medication that precisely treats EoE in children.
Years of research producing results
Rothenberg and Margaret Collins, MD, Division of Pathology and Laboratory Medicine at Cincinnati Children’s, were co-authors on both studies published in the NEJM. They have been studying EoE for decades and have been active since the early days in evaluating both drugs.
Julie Caldwell, PhD, Division of Allergy and Immunology at Cincinnati Children’s, was a co-author on the benralizumab study.
What’s next for doctors, researchers and people with EoE?
In the short term, Rothenberg says that patients should consider dupilumab, but not benralizumab, or continue (or return to) other treatments that have been helpful. Dietary elimination therapy to avoid offending food triggers has been used for years but can be tough to follow. Various medications have helped some patients manage symptoms, including proton-pump inhibitors and steroid treatments. A few newer approaches are still being studied in other clinical trials.
Longer term, Rothenberg and other scientists are looking farther “upstream” from eosinophils in the body’s natural cascade of immune system responses to find other ways to stop, slow, or prevent the damage caused by EoE.
Until such work produces new options, Rothenberg also recommends that clinicians rely less upon eosinophil levels as the sole feature to gauge the severity of EoE. Other tests such as endoscope exams and collecting tissue samples for microscope analysis appear more likely to provide meaningful information.
“We need a more relevant biomarker to measure clinical response in eosinophilic esophagitis,” Rothenberg says. “That is certainly one of our main research goals as we move forward.”
About these studies
The MESSINA clinical trial was supported by AstraZenica. The dupilumab clinical trial was supported by Sanofi and Regeneron Pharmaceuticals.
Journal
New England Journal of Medicine
DOI
10.1056/NEJMoa2313318
Method of Research
Randomized controlled/clinical trial
Subject of Research
People
Article Title
Eosinophil Depletion with Benralizumab for Eosinophilic Esophagitis
Article Publication Date
26-Jun-2024
COI Statement
Study supported by AstraZeneca




