The lanthanide metals are unique resources with applications ranging from magnets and catalysts to cancer treatments. Their future availability hangs on the creation of better strategies to separate lanthanide groups into individual elements because conventional approaches are laborious, costly and generate waste.
Credit: Alex Ivanov/ORNL, U.S. Dept. of Energy
The lanthanide metals are unique resources with applications ranging from magnets and catalysts to cancer treatments. Their future availability hangs on the creation of better strategies to separate lanthanide groups into individual elements because conventional approaches are laborious, costly and generate waste.
“Our approach is flexible and can be tailored to select specific lanthanides for a faster route to separating adjacent elements,” said Oak Ridge National Laboratory’s Santa Jansone-Popova.
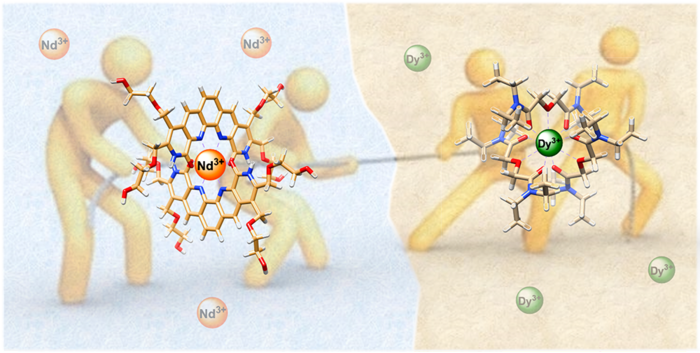
ORNL scientists combined two ligands, or metal-binding molecules, to target light and heavy lanthanides simultaneously for exceptionally efficient separation.
Solvent extraction leverages the separating feature of oil-water mixtures and typically employs ligands that guide targeted materials from water to oil. The new strategy pairs an oil-loving ligand that seeks heavier lanthanides with a water-loving counterpart that targets lighter elements. The tug-of-war match-up pulls apart lanthanides that are especially difficult to divide.
“Fundamental discoveries such as this one can advance economical and environmentally responsible separations strategies,” Jansone-Popova said.
UT-Battelle manages ORNL for the Department of Energy’s Office of Science, the single largest supporter of basic research in the physical sciences in the United States. The Office of Science is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.
Journal
JACS Au
DOI
10.1021/jacsau.2c00671
Article Publication Date
25-Jan-2023



