Researchers led by Genshiro Sunagawa at the RIKEN Center for Biosystems Dynamics Research (BDR) in Japan have shown that an animal’s stem cells possess the same level of cold resistance as the animal itself. Published August 17 in Cell Reports, the study focuses on mice with different hibernation-like characteristics, showing that those with the best resistance to cold temperatures have stems cells that generate energy differently than others. Beyond these immediate findings, the study establishes mouse stem cells as a practical model system for further research into organ preservation and even human hibernation.
Credit: RIKEN
Researchers led by Genshiro Sunagawa at the RIKEN Center for Biosystems Dynamics Research (BDR) in Japan have shown that an animal’s stem cells possess the same level of cold resistance as the animal itself. Published August 17 in Cell Reports, the study focuses on mice with different hibernation-like characteristics, showing that those with the best resistance to cold temperatures have stems cells that generate energy differently than others. Beyond these immediate findings, the study establishes mouse stem cells as a practical model system for further research into organ preservation and even human hibernation.
Whether it’s to move organs for transplant or put the brain in temporary stasis after a stroke, cold temperatures eventually cause irreparable damage. At the same time, hibernating animals can survive for months with low body temperature because their metabolism becomes extremely slow. Sunagawa and his team at the RIKEN BDR Laboratory for Hibernation Biology are trying to understand how an animal, or just its organs, can survive under these conditions. Rather than studying wild hibernators, they are using laboratory mice because genetic variables can be controlled. The goal of the new experiments was to determine if mouse stem cells can be used to study organ preservation and hibernation. This would open up the field to experimental systems already developed for studying stem cells, as well as reduce the need for using live animals in the research.
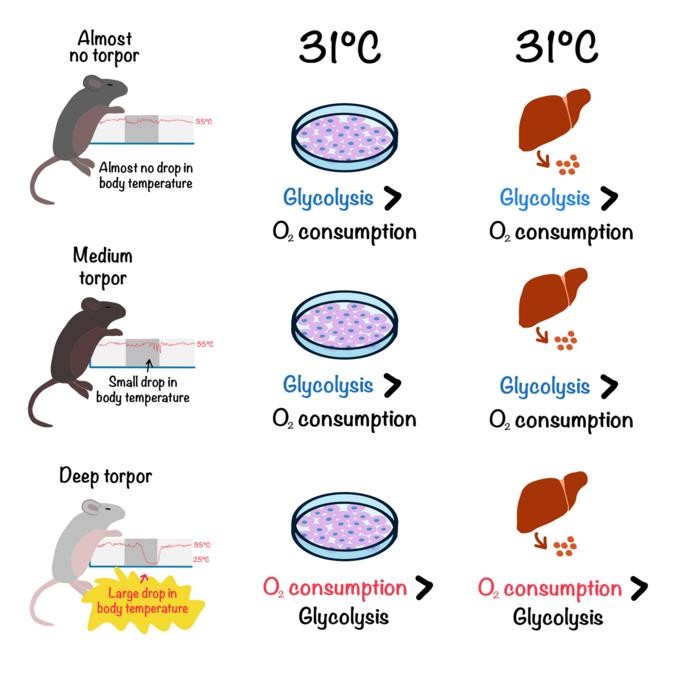
Although mice do not actually hibernate, they can enter a short one-hour hibernation period called torpor. The researchers tested strains of inbred laboratory mice and characterized torpor by the lowest body temperature and the lowest oxygen use the mice achieved. They found one strain with shallow torpor, one with deep torpor, and one in between. They then established embryonic stem cell lines from each mouse strain and examined cell metabolism at around mouse body temperature (37°C / 98.6°F) and a cold temperature (31°C / 88.6°F).
The energy that cells, and therefore animals, need to survive is produced in two ways. One way, called glycolysis, involves breaking down sugar molecules. This process does not require oxygen, and we can feel its byproduct in our muscles when we exercise and use up our available oxygen. The other way, called oxidative phosphorylation or OXPHOS, takes place in the mitochondria and does require oxygen. The researchers found that stem cells from the shallow- and medium-torpor mice switched to glycolysis at the colder temperature, while the stem cells from the deep-torpor mice did not. Those stem cells maintained a high rate of oxygen consumption at all temperatures, meaning that somehow, oxygen remained available despite the cold. Further tests revealed that the way energy is generated using oxygen in the mitochondria of the deep-torpor stem cells differed from the others, with the oxygen coming from an outside source.
Lastly, the researchers asked whether what they learned from the stem cells was actually relevant in adult mice. They examined liver tissue from each of the mouse lines and found that as in the stem cells, the tissue from the deep-torpor mice did not rely on glycolysis in the cold and maintained a high degree of oxygen use. So, even when not undergoing torpor, tissue from these mice remained better preserved at lower temperatures.
“We’ve proven that the distinct responses to lower temperatures, unique to each strain, are preserved even at the cellular level,” says Sunagawa. “This provides a huge opportunity to conduct in vitro studies of cold tolerance in tissues from torpor-capable animals.” Eventually, they hope to be able to induce similar cold-resistance in tissue from any animal.
“In the long-term, insights from this system will help us develop ways to implement human hibernation or human organ cryopreservation.”
Journal
Cell Reports
DOI
10.1016/j.celrep.2023.112954




