Credit: Tamae Seo, et al. Journal of the American Chemical Society. March 30, 2021
Scientists from Hokkaido University have developed a rapid, efficient protocol for cross-coupling reactions, vastly expanding the pool of chemicals that can be used for the synthesis of useful organic compounds.
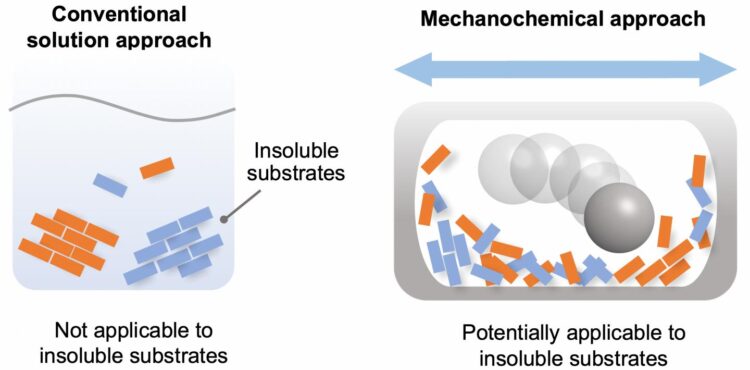
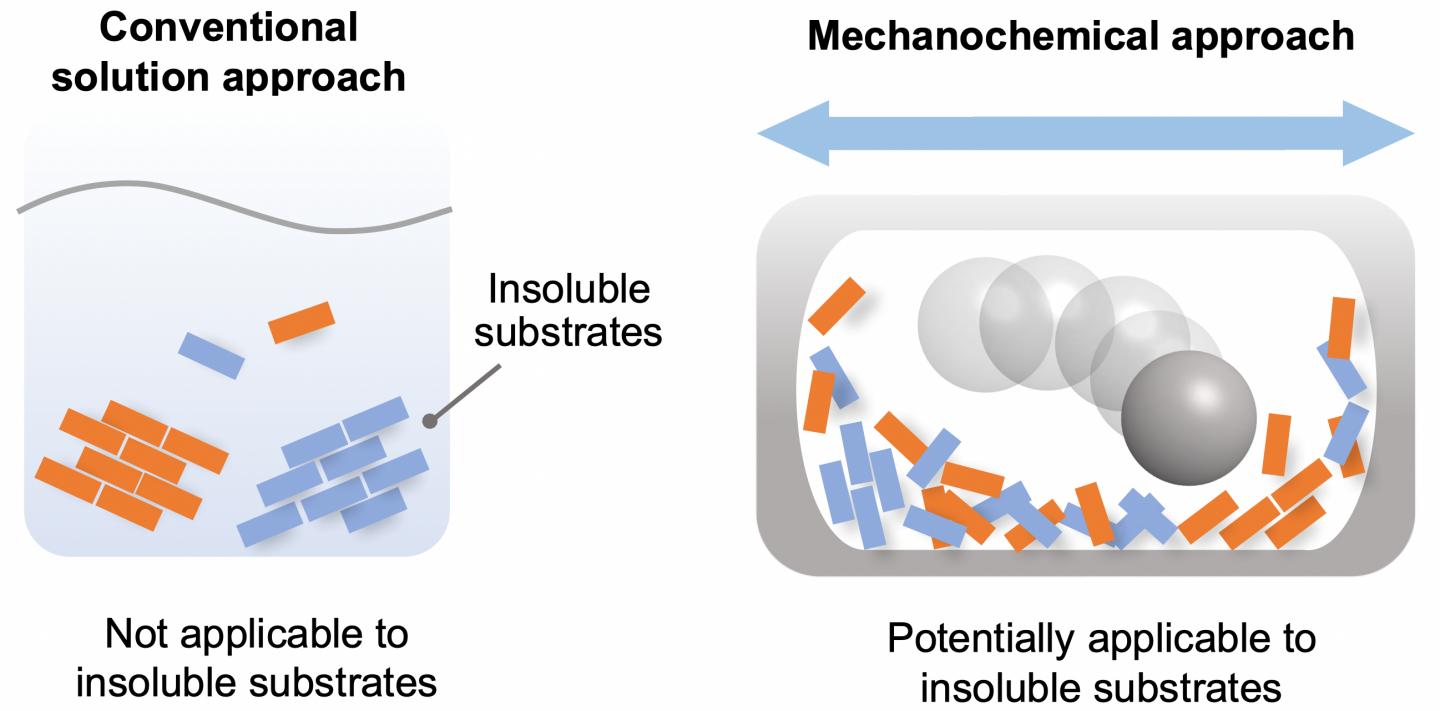
Chemical reactions are a vital process in the synthesis of products for a diversity of purposes. For the most part, these reactions are carried out in the liquid phase, by dissolving the reactants in a solvent. However, there are a significant number of chemicals that are partially or completely insoluble, and thus have not been used for synthesis. The starting materials required for the synthesis of many cutting-edge organic materials–such as organic semiconductors and luminescent materials–are often poorly soluble, leading to problems in solution-based synthesis. Therefore, the development of a solvent-independent synthetic approach to overcome these long-standing solubility issues in organic synthesis is highly desired to synthesize new valuable organic molecules.
In recent years, synthetic techniques using ball milling have been used to carry out solvent-free reactions in the solid phase. It has been proposed that the use of ball milling would potentially overcome the aforementioned solubility issues in synthetic chemistry, but a systematic study for such purpose has never been carried out.
A team of four scientists from Hokkaido University’s Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), led by Associate Professor Koji Kubota and Professor Hajime Ito, have developed a rapid, efficient, solvent-free protocol for Suzuki?Miyaura cross-coupling reaction of insoluble aryl halides. The protocol was published in the Journal of the American Chemical Society.
Aryl halides are popular starting materials for the synthesis of organic functional molecules, primarily by the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction — for which Hokkaido University’s Professor Emeritus Akira Suzuki shared the 2010 Nobel Prize in Chemistry. Although the cross-coupling reactions have been employed for the synthesis of a wide range of valuable molecules, insoluble aryl halides are not suitable substrates because Suzuki-Miyaura cross-coupling reactions have primarily been carried out in solution.
Given this limitation, the scientists focused on the development of an efficient solid-state Suzuki-Miyaura cross-coupling of a number of extremely unreactive insoluble aryl halides. The key equipment consisted of a ball mill, for mixing the reactants; a heat gun, to increase the temperature at which the reactions took place; and the use of a catalytic system composed of palladium acetate (the catalyst), SPhos (a high-performance ligand for Suzuki?Miyaura cross-coupling reactions) and 1,5-cyclooctadiene (dispersant and stabilizer).
The capstone of this study was the application of the solvent-free solid-state reaction to mostly-insoluble aryl halides. These reactants did not yield any products in conventional solution-based reactions. The solid-state reactions using the high-temperature ball milling, however, gave the desired products. Importantly, the team discovered a new strong photoluminescence material prepared from insoluble pigment violet 23.
“The high-temperature ball-milling technique and our catalytic system are essential for these cross-coupling reactions of insoluble aryl halides, and the protocol we have developed expands the diversity of organic molecules derived from insoluble starting materials,” says Koji Kubota.
###
Media Contact
Sohail Keegan Pinto
[email protected]
Original Source
https:/
Related Journal Article
http://dx.