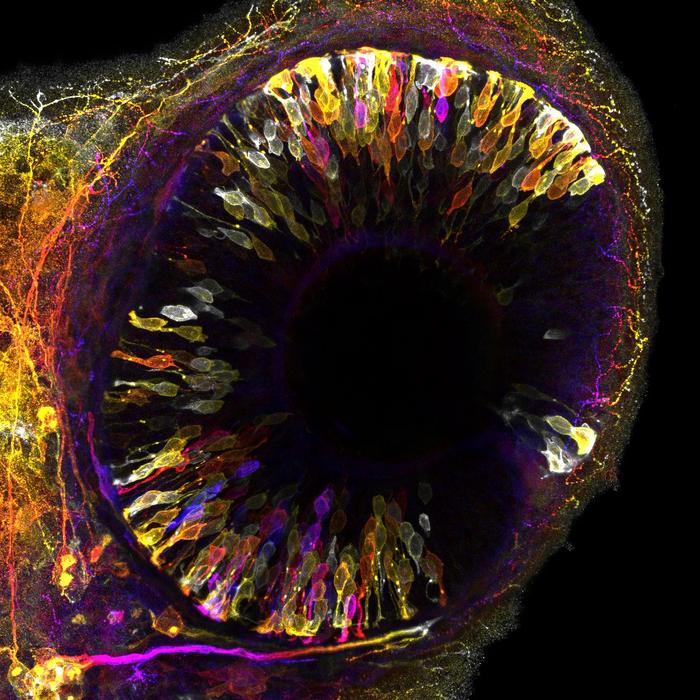
To function properly, organs require a precise number of cells and a functional architecture, which are established during embryogenesis. Embryos are proficient multitaskers; they grow, and acquire shape and functional architecture all at once. Despite a lot of research on embryo development, scientists do not yetfully grasp how embryos orchestrate all these different tasks in space and time to ensure the formation of healthy organs. This was the central question of the study led by Caren Norden (group leader) and MauricioRocha-Martins (postdoctoral researcher). The research team, that also involved computer scientists, usedcutting-edge technology to explore how the vertebrate retina copes with the challenges of growing profuselywhile, at the same time, remodeling tissue architecture. The retina of zebrafish embryos and human retinal organoids—mini- retina-like structures in a dish grown from human cells—were used as model systems because they both offer unique advantages due to their small size and high translucency, allowing real-time observation of tissue organization and growth. Advanced microscopy techniques, such as light-sheet microscopy and state-of-the-art image restoration based on deep learning, provided unprecedented insight into the cellular behaviors involved.
Credit: ©Mauricio Rocha-Martins, IGC 2023.
To function properly, organs require a precise number of cells and a functional architecture, which are established during embryogenesis. Embryos are proficient multitaskers; they grow, and acquire shape and functional architecture all at once. Despite a lot of research on embryo development, scientists do not yetfully grasp how embryos orchestrate all these different tasks in space and time to ensure the formation of healthy organs. This was the central question of the study led by Caren Norden (group leader) and MauricioRocha-Martins (postdoctoral researcher). The research team, that also involved computer scientists, usedcutting-edge technology to explore how the vertebrate retina copes with the challenges of growing profuselywhile, at the same time, remodeling tissue architecture. The retina of zebrafish embryos and human retinal organoids—mini- retina-like structures in a dish grown from human cells—were used as model systems because they both offer unique advantages due to their small size and high translucency, allowing real-time observation of tissue organization and growth. Advanced microscopy techniques, such as light-sheet microscopy and state-of-the-art image restoration based on deep learning, provided unprecedented insight into the cellular behaviors involved.
The researchers observed that an entire population of neurons, photoreceptors, temporarily relocates away from the zone of the tissue where they reside and must fulfill their function (Fig. 1). This active movement creates space for incoming progenitor cells that divide in this area and thereby produce more cells that latercontribute to the neuronal retina. Blockage of the movements of photoreceptors leads to congestion, forcing progenitor cells to divide in wrong place which in turn causes tissue malformation. Thus, by transiently moving away, neurons avoid interference with progenitor cells to ensure harmonious organ development.
To Mauricio Rocha-Martins, the first author of the study, “This is a curious migration phenomenon, in whichneurons move away just to then move back, ending up where they started. It highlights that neuronalmigration, as opposed to what was previously believed, does not only move neurons to their correct location but can also play a direct role in the coordination of organ development”.
The implications of this research extend beyond the field of retinal development. Simultaneous growth andacquisition of functional architecture is a hallmark of most developing organs; the new findings offer the possibilityto investigate whether other developing organs employ similar strategies. Moreover, it is known that defects in neuronal migration can cause severe brain malformations in humans. The findings that failed migration of neurons can have deleterious consequences beyond the positioning of neurons points to the importance of examining the interactions between cells to fully understand the causes of human developmental disorders.
The study was supported by the MPI-CBG, the FCG-IGC, the German Research Foundation (NO 1069/5-1), and an ERC Consolidator Grant (H2020 ERC-2018-CoG-81904).
Journal
Nature
DOI
10.1038/s41586-023-06392-y
Article Title
Bidirectional neuronal migration coordinates retinal morphogenesis by preventing spatial competition
Article Publication Date
9-Aug-2023




