Many tumor cells mist themselves with a protective perfume that disables the immune system. But a drug already approved for other purposes can apparently render this weapon harmless. This is shown in a study by the University of Bonn and the University Medical Center Hamburg-Eppendorf, which has now appeared in the Journal for ImmunoTherapy of Cancer. The researchers now want to further optimize the compound. In the medium term, this could pave the way for new anti-cancer drugs.
Credit: Figure: Laura Schäkel/University of Bonn – created with BioRender.com
Many tumor cells mist themselves with a protective perfume that disables the immune system. But a drug already approved for other purposes can apparently render this weapon harmless. This is shown in a study by the University of Bonn and the University Medical Center Hamburg-Eppendorf, which has now appeared in the Journal for ImmunoTherapy of Cancer. The researchers now want to further optimize the compound. In the medium term, this could pave the way for new anti-cancer drugs.
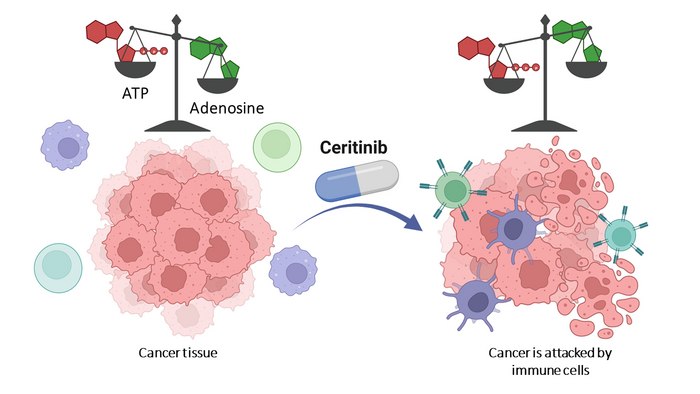
Many cancer cells surround themselves with a dense cloud of adenosine. On the one hand, the molecule suppresses the immune system. At the same time, it stimulates the formation of new blood vessels that supply the tumor with oxygen and nutrients. It also ensures that the malignant cells migrate to other organs and form metastases there.
Adenosine is produced from adenosine triphosphate, or ATP for short. Tumor cells secrete large amounts of it. They carry various enzymes on their surface that then convert the ATP to adenosine in several steps. One of these is known as CD39. “It catalyzes the first of the conversion steps,” explains Prof. Dr. Christa Müller from the Institute of Pharmacy at the University of Bonn. “If CD39 is inhibited, hardly any adenosine is produced.”
Around the globe, pharmaceutical researchers are therefore searching for an active ingredient that slows down CD39. Because without adenosine, tumors would no longer be protected from the immune system. “Instead, ATP would accumulate around the cancer cells, which would actually stimulate the immune response,” says Müller. “So the body’s own defenses would not be suppressed; on the contrary, they would be turned on extra sharp.”
50 approved active substances scrutinized
So far, the search has been largely unsuccessful. The Bonn research group therefore pursued a new search strategy in the study: “There are other enzymes in the body than CD39 that also process ATP,” explains Laura Schäkel. The collaborator of Prof. Müller carried out many of the central experiments in the study. “These include, for example, the so-called protein kinases. The nice thing is that there are already approved drugs that inhibit protein kinases. We now looked at whether they also work against CD39.”
At the start of the study, there were a total of 50 different agents approved for certain diseases that inhibit protein kinases. The research group examined all of them. With success: “One of the substances, ceritinib, also blocks the conversion of ATP by CD39,” Schäkel is pleased to report. “We were able to show this not only in the test tube, but also in cultures with so-called triple-negative breast cancer cells. These are extremely difficult to treat – they usually hardly respond to therapies.”
Nevertheless, Christa Müller does not think it makes sense to simply administer ceritinib as a CD39 inhibitor in certain cancers. “After all, the active ingredient is primarily directed against a different group of enzymes; it would therefore have undesirable side effects,” she says. “We now want to modify it so that it hardly inhibits protein kinases at all and instead slows down CD39 even more.”
Use only in patients for whom it is worthwhile
Such an optimized active ingredient could also be combined with other therapeutic agents. “Classic cytostatics usually massively weaken the immune system; CD39 blockers, on the other hand, would activate it,” says Prof. Müller, who is also a member of the Transdisciplinary Research Areas (TRA) “Building Blocks of Matter” and “Life and Health.” “In combination, therefore, the drugs could possibly have a significantly greater effect.”
Before use, moreover, it would first be possible to measure whether the cancer cells of affected patients actually carry a lot of CD39 on their surface. “Because only then would treatment with CD39 inhibitors make sense,” says Müller. “So you would tailor the administration to the individual patient. This personalization of therapies for the purpose of enhancing efficiency is becoming increasingly important in medicine.”
Participating institutions and funding:
The University of Bonn, the University Medical Center Hamburg-Eppendorf and the Université Laval in Quebec, Canada, were involved in the study. The study was supported by the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Publication: Schäkel L, Mirza S, Winzer R, et al: Protein kinase inhibitor ceritinib blocks ectonucleotidase CD39 – a promising target for cancer immunotherapy, Journal for ImmunoTherapy of Cancer, DOI: 10.1136/jitc-2022-004660
Contact:
Prof. Dr. Christa E. Müller
Pharmaceutical Institute of the University of Bonn
Department of Pharmaceutical and Medicinal Chemistry
Phone +49 (0)228 732301
E-mail: [email protected]
Journal
Journal for ImmunoTherapy of Cancer
DOI
10.1136/jitc-2022-004660
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Protein kinase inhibitor ceritinib blocks ectonucleotidase CD39 – a promising target for cancer immunotherapy
Article Publication Date
18-Aug-2022




