Credit: ©Batista Rocha/IMBA
The Wnt signalling pathway has been studied for decades, still it holds surprises in store. Bon-Kyoung Koo, group leader at IMBA – Institute of Molecular Biotechnology of the Austrian Academy of Sciences and Tadasuke Tsukiyama at the Hokkaido University have now uncovered a new and unexpected role for a key component of the Wnt pathway, Casein Kinase-1, in regulating the pathway at the plasma membrane. This is the result of a study published today in Nature Communications, and broadens our understanding of the regulatory loops controlling the Wnt pathway, a pathway associated with stem cell maintenance, cell proliferation and cancer.
In the Wnt pathway, Casein Kinase-1 is well-known as a part of the destruction complex. In the steady-state, when no Wnt signal is present, this complex destines the downstream mediator ?-catenin for constant degradation. When a Wnt signal reaches the cell, the Wnt receptor Frizzled inhibits the destruction complex. This allows ??-catenin to enter the nucleus, where it sets downstream responses in motion.
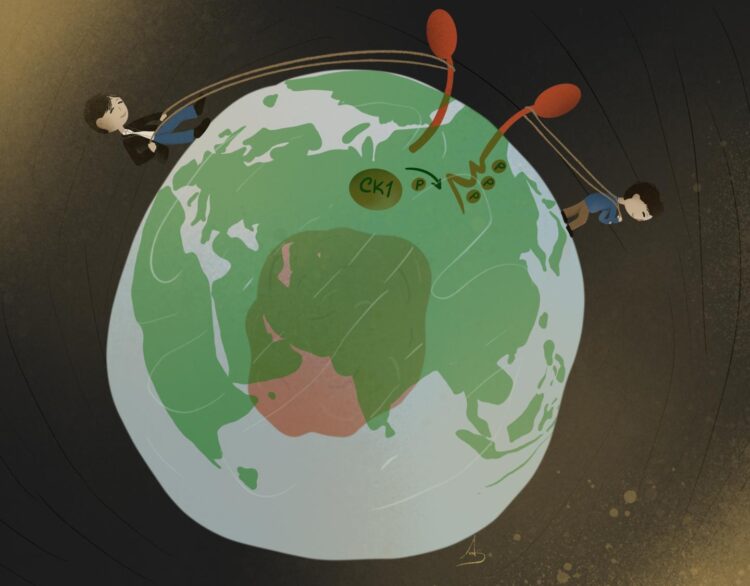
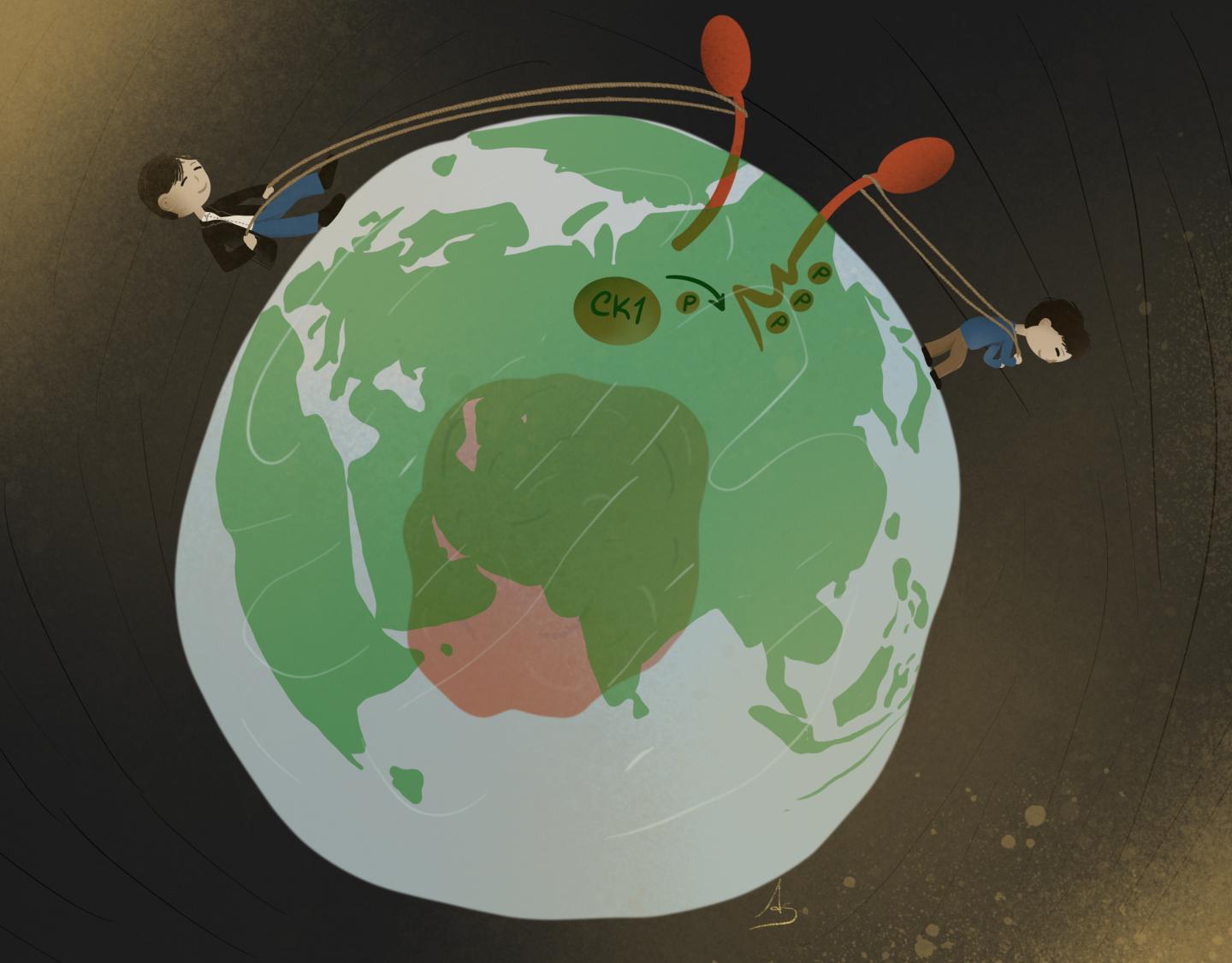
Casein Kinase-1 responsible for phosphoswitch of RNF43
In the newly published study, Tadasuke Tsukiyama and Bon-Kyoung Koo find that Casein Kinase-1 also regulates Wnt signalling at the plasma membrane. At the plasma membrane, the ubiquitin ligase RNF43 marks the Wnt receptor Frizzled for degradation, effectively shutting off the Wnt signalling pathway. The researchers discovered that Casein Kinase-1 triggers the switch for RNF43: When Casein Kinase-1 phosphorylates RNF43, RNF43 is activated and marks Frizzled with ubiquitin for degradation. When Casein Kinase-1 does not phosphorylate RNF43, RNF43 is inactive and signalling via Frizzled can continue. “We find that Casein Kinase-1 has an essential function in activating RNF43. With our work, we are effectively reintroducing Casein Kinase-1 to the field, defining a new role for this well-known regulator”, Bon-Kyoung Koo explains.
This new understanding could lead to a novel approach for reining the Wnt pathway in cancer cells. Tsukiyama and Koo found that a mutation in RNF43’s extracellular domain interrupts its function in negative feedback regulation, the tumour suppressor function of RNF43. This mutation changes RNF43 into an oncogenic form that abnormally enhances Wnt signalling. The researchers found that mimicking the phosphorylation, by adding negatively charged residues to the mutant RNF43, can revert it back to a functional tumour suppressor. With this mimicked phosphoswitch, the mutant RNF43 was again able to inhibit Frizzled. “Some patients carry a mutation in RNF43’s extracellular domain. We hope that, once we know how to mimic phosphorylation in cells, this phosphorylation would revive the RNF43 tumour suppressor, enabling it to again control the Wnt pathway”, Bon-Kyoung Koo adds.
###
About IMBA
IMBA – Institute of Molecular Biotechnology – is one of the leading biomedical research institutes in Europe focusing on cutting-edge stem cell technologies, functional genomics, and RNA biology. IMBA is located at the Vienna BioCenter, the vibrant cluster of universities, research institutes and biotech companies in Austria. IMBA is a subsidiary of the Austrian Academy of Sciences, the leading national sponsor of non-university academic research. The stem cell and organoid research at IMBA is being funded by the Austrian Federal Ministry of Science and the City of Vienna.
Media Contact
Caterina Purini
[email protected]
Related Journal Article
http://dx.