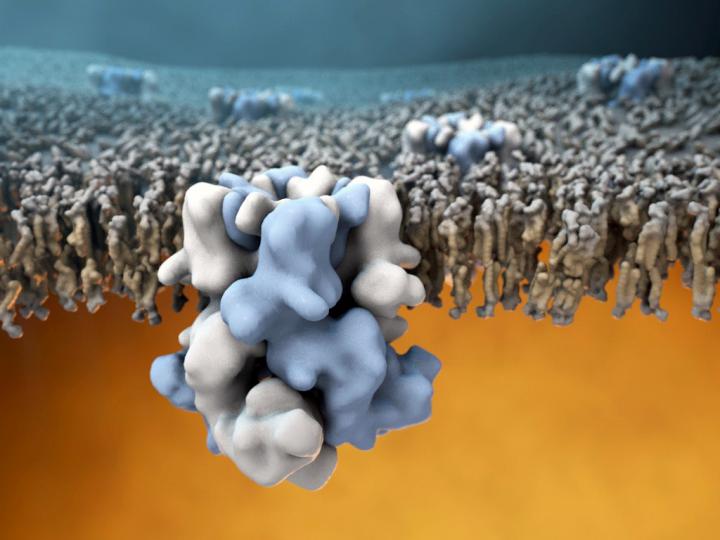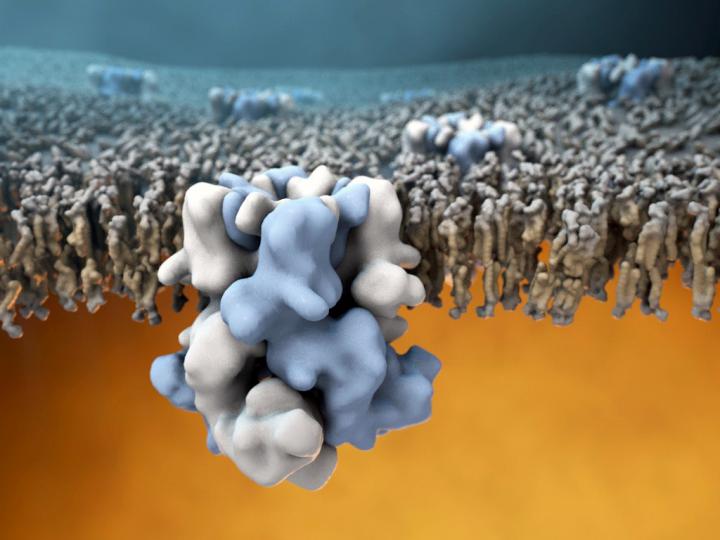
Credit: Image by Gabe Lander and Graham Johnson
LA JOLLA, Calif. – Dec. 7, 2017 – A team of researchers from The Scripps Research Institute (TSRI) and Duke University has made the first determination of the atomic structure of Transient Receptor Potential Melastatin 8 (TRPM8), a molecular sensor in nerve ends that detects cold temperatures as well as menthol and other chemicals that induce cold sensations.
This finding should boost ongoing efforts by scientists to target TRPM8 therapeutically. Drug compounds that interact with the cold sensor–as menthol-containing salves already do–may be able to treat some forms of chronic pain and inflammation, migraines and even cancers.
"Knowing the atomic structure of TRPM8 and how it reacts to cold, menthol and other stimuli should help in the design of potent and selective new drugs targeting this sensor," said study leader Gabriel C. Lander, Ph.D., associate professor at TSRI, who co-led the study with Dr. Seok-Yong Lee at Duke University School of Medicine.
The discovery, published on Dec. 7, 2017 in the journal Science, is also a significant technical feat. Ever since the cold-sensing protein was first identified in 2002, teams around the world have tried but failed to determine the atomic structure of TRPM8 using X-ray crystallography, traditionally the go-to method for solving large-protein structures. Obtaining a high resolution structure of TRPM8 has posed a major challenge for structural biologists in part because of the instability of the channel when isolated from its native environment in the cellular membrane. Without membrane support, TRPM8 has a tendency to lose its structural integrity, making the target very difficult to study. The TRPM8 sensor structure is also relatively complex, being composed of four identical copies of the protein encoded by the TRPM8 gene.
For this project, Lander and co-first author Mengyu Wu, a graduate student in his laboratory, opted instead to use cryo-electron microscopy (cryo-EM), a structure determination method that is increasingly preferred for difficult structural studies. Lee and his team began by screening TRPM8 proteins from more than a dozen different animal species, including humans, mice, and birds, to find one that was likely to be "best behaved" for a cryo-EM study. They settled on the TRPM8 protein from a bird called the collared flycatcher.
"All evidence points to the flycatcher TRPM8 working in the same way as mammalian TRPM8s, so we're confident that our structural analysis will translate directly to the human form of this sensor," Lander said.
The scientists faced many hurdles due to the inherent instability of TRPM8 outside of its native membrane environment. "Even within a single day of shipping the samples from Duke to TSRI, the protein complex would start to fall apart," said Wu. "The Lee lab strategically added a few stabilizing mutations to the protein so that it would be less prone to degradation ." Ying Yin, a graduate student in the Lee laboratory, also went back and meticulously screened through several purification conditions to confer additional stability to the sample.
The protein also behaved differently from most of the samples that the Lander lab typically works on for electron microscopy, and it took the researchers over a year to identify the right conditions to image this challenging biomolecule.
"We had to throw out the rulebook and rethink the usual approach to solving this kind of structure," said Lander.
Through these adaptations, the researchers were able to obtain the first structural glimpse of TRPM8 at an overall resolution of about 4 Angstroms (0.4 billionths of a meter). The resulting atomic model contained some surprises too. "Other groups have hypothesized about the structure of TRPM8 and how it interacts with binding partners such as menthol, but what we found was that virtually all these educated guesses were pretty far off," Lander said. In particular, the binding pocket for menthol turned out to be in an unexpected location, different from the ligand binding location in other TRP sensors.
"One thing this structure tells us is that TRP sensors do not all work in the same way, and so I expect that we're going to discover many new sensor mechanisms as we study more of these TRP structures," Lander said.
TRPM8 is of interest to the pharmaceutical industry in part because of the analgesic, anti-inflammatory effects it can have when activated. Moreover, variants of its gene have been linked to a predisposition to migraines, and scientists have shown that manipulating TRPM8 can cause migraine-like pain in animals.
"TRPM8 also is abnormally expressed in some prostate, breast and other cancers, making it a potential chemotherapeutic target," Wu said.
Although TRPM8 is best known as a peripheral-nerve sensor of moderately cold temperatures (below about 25° C) and of cold-sensation molecules such as menthol, it is also found within many other normal tissues, even deep within the body, and its functions in those tissues remain largely unclear. A detailed understanding of TRPM8's structural interaction with its natural binding partners should lead to the development of better molecular probes that can help scientists reveal its various functions.
The Lander and Lee Laboratories are now working to better understand how TRPM8 interacts with menthol and other therapeutic binding-partner molecules.
###
Additional authors of the study, "Structure of the cold- and menthol-sensing ion channel TRPM8," were co-first author Ying Yin, and Lejla Zubcevic and William F. Borschel, all of the Lee Laboratory.
Funding was provided by the National Institutes of Health (grants R35NS097241 and DP2EB020402).
About The Scripps Research Institute
The Scripps Research Institute (TSRI) is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. TSRI is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs more than 2,500 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists–including two Nobel laureates and 20 members of the National Academies of Science, Engineering or Medicine–work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation. In October 2016, TSRI announced a strategic affiliation with the California Institute for Biomedical Research (Calibr), representing a renewed commitment to the discovery and development of new medicines to address unmet medical needs. For more information, see http://www.scripps.edu.
Media Contact
Madeline McCurry-Schmidt
[email protected]
858-784-9254
@scrippsresearch
http://www.scripps.edu