They have just published the results of their study in the journal Frontiers in Bioengineering and Biotechnology. During normal breathing, the diaphragm descends below the lungs with each breath. This causes the lungs to expand inside the chest, creating a negative pressure, or vacuum, in the lungs. To compensate for this negative pressure, air automatically flows into the lungs and the person breathes in. Mechanical ventilation involves pumping air into the lungs through a tube. The lungs then expand due to this positive pressure. “We assume that this positive pressure causes a slight compression of the lung tissue, whereas during normal breathing the lung is ‘pulled’ from the outside in order to create the expansion,” explains physicist Professor Mareike Zink, who conducted the interdisciplinary study on the physics of the premature lung together with her colleague Dr Mandy Laube from the neonatology research laboratory at the Faculty of Medicine.
“In our experiments, we studied foetal lung tissue under tensile and compressive stress to explore differences in tissue mechanics in the premature lung,” Mareike Zink reports. The experiments showed that the lung tissue deformed completely elastically under tension, as occurs during normal breathing. When subjected to pressure, however – as occurs with mechanical ventilation – viscoelastic deformation of the lungs was observed. This means that although the tissue returns to its original state after deformation, at the molecular level, there are already structural changes that indicate irreversible tissue damage.
“Furthermore, our results show that lung cell function is impaired under pressure. Even low pressure, as is common in mechanical ventilation, can result in structural units on the cell surface, which are important in the transport of molecules and water, for example, no longer being able to perform their function,” explains Mandy Laube.
The two scientists draw the following conclusion: for some premature infants, mechanical ventilation is the only treatment to ensure survival. Nevertheless, there is a risk of complications due to the altered mechanical properties of premature lungs compared to adults. Future therapeutic strategies should therefore consider the influence of physical forces on tissues and cells, and limit pressure increases in the lungs so as to minimise the risk of damage. “Since it has also been observed in ventilated Covid-19 patients that mechanical ventilation may result in further lung damage, we postulate that here, too, the damaged lung can be more easily overstretched by the positive pressure and that lung cell function stops or changes more quickly under increased pressure,” Mareike Zink concludes.
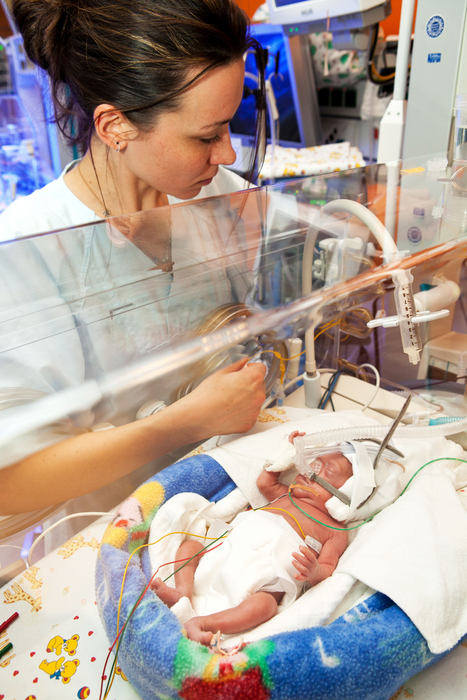
Credit: Stefan Straube/University of Leipzig Medical Center
They have just published the results of their study in the journal Frontiers in Bioengineering and Biotechnology. During normal breathing, the diaphragm descends below the lungs with each breath. This causes the lungs to expand inside the chest, creating a negative pressure, or vacuum, in the lungs. To compensate for this negative pressure, air automatically flows into the lungs and the person breathes in. Mechanical ventilation involves pumping air into the lungs through a tube. The lungs then expand due to this positive pressure. “We assume that this positive pressure causes a slight compression of the lung tissue, whereas during normal breathing the lung is ‘pulled’ from the outside in order to create the expansion,” explains physicist Professor Mareike Zink, who conducted the interdisciplinary study on the physics of the premature lung together with her colleague Dr Mandy Laube from the neonatology research laboratory at the Faculty of Medicine.
“In our experiments, we studied foetal lung tissue under tensile and compressive stress to explore differences in tissue mechanics in the premature lung,” Mareike Zink reports. The experiments showed that the lung tissue deformed completely elastically under tension, as occurs during normal breathing. When subjected to pressure, however – as occurs with mechanical ventilation – viscoelastic deformation of the lungs was observed. This means that although the tissue returns to its original state after deformation, at the molecular level, there are already structural changes that indicate irreversible tissue damage.
“Furthermore, our results show that lung cell function is impaired under pressure. Even low pressure, as is common in mechanical ventilation, can result in structural units on the cell surface, which are important in the transport of molecules and water, for example, no longer being able to perform their function,” explains Mandy Laube.
The two scientists draw the following conclusion: for some premature infants, mechanical ventilation is the only treatment to ensure survival. Nevertheless, there is a risk of complications due to the altered mechanical properties of premature lungs compared to adults. Future therapeutic strategies should therefore consider the influence of physical forces on tissues and cells, and limit pressure increases in the lungs so as to minimise the risk of damage. “Since it has also been observed in ventilated Covid-19 patients that mechanical ventilation may result in further lung damage, we postulate that here, too, the damaged lung can be more easily overstretched by the positive pressure and that lung cell function stops or changes more quickly under increased pressure,” Mareike Zink concludes.
Journal
Frontiers in Bioengineering and Biotechnology
DOI
10.3389/fbioe.2022.964318
Method of Research
Experimental study
Subject of Research
Human tissue samples
Article Title
Mechanical properties of the premature lung: From tissue deformation under load to mechanosensitivity of alveolar cells
Article Publication Date
16-Sep-2022




