Researchers at the RIKEN Center for Brain Science (CBS) in Japan have discovered a set of related mutations that lead to intracranial aneurysms—weakened blood vessels in the brain that can burst at any time. The mutations all appear to act on the same biological signaling pathway, and the researchers report the first ever pharmaceutical treatment in a mouse model, which works by blocking this signal. The study was published in Science Translational Medicine on June 14.
Credit: RIKEN
Researchers at the RIKEN Center for Brain Science (CBS) in Japan have discovered a set of related mutations that lead to intracranial aneurysms—weakened blood vessels in the brain that can burst at any time. The mutations all appear to act on the same biological signaling pathway, and the researchers report the first ever pharmaceutical treatment in a mouse model, which works by blocking this signal. The study was published in Science Translational Medicine on June 14.
About 5% of the population have unruptured intracranial aneurysms in blood vessels on the surface of the brain. Despite being ballooned arteries with weakened walls, intracranial aneurysms often go undetected — until a rupture leads to deadly bleeding around the brain. Even when they are detected in advance, the only currently available treatment options involve surgery, which has its own set of risks, especially if the aneurysm is in a sensitive location. Finding other, non-surgical options is thus a high priority, and research into the origin of intracranial aneurysms has led the RIKEN CBS team to one such potential treatment.
Intracranial aneurysms actually come in two types called intracranial fusiform aneurysms (IFAs) and intracranial saccular aneurysms (ISAs ), with about 90% being the ISA variety. Previous research reported mutations in IFA arteries, but the origins of the more common ISA type remain unclear. To address this issue, the RIKEN team sequenced the entire exomes—all protein-encoding pieces of DNA— in cells that made up 65 aneurysmal arteries and 24 normal arteries. Along with subsequent deep-targeted sequencing, they found that six genes were common among IFAs and ISAs and never appeared in non-aneurysmal arteries, while 10 others appeared only in either IFAs or ISAs. While several factors, such as age, hypertension, and alcohol consumption, increase the risk of intracranial aneurysms, project leader Hirofumi Nakatomi from RIKEN CBS notes, “the unexpected finding that greater than 90% of aneurysms had mutations in a common set of 16 genes indicates that somatic mutation could be the major trigger in most cases.”
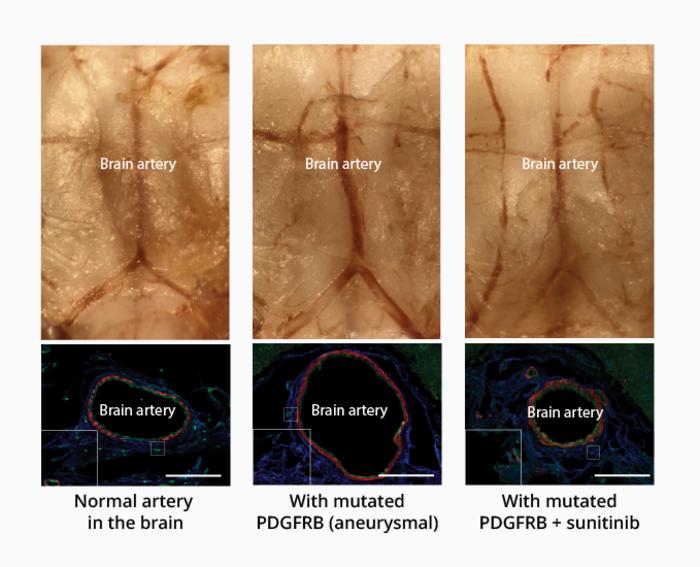
Further testing showed that mutations to all six of the genes common to IFAs and ISAs triggered the same NF-κB biological signaling pathway. The next step was to learn more about the mutations and try to block the abnormal signaling. First, they showed that mutations to one of the six genes, PDGDRB, could be traced along different layers within samples of human aneurysms. Then, after linking the PDGDRB mutation with faster cell migration and inflammation in cultured cells, they discovered that these effects could be blocked with sunitinib, a drug that prevents the changes to PDGDRB that allow signaling.
Next, they created a mouse model of intracranial aneurysm by using an adenovirus to insert mutant PDGFRB into the basilar artery at the base of the brain. After a month, the size of the artery had doubled in diameter and become very weak. As in the cultured cells, the effect of the mutant gene was blocked when the mice were given sunitinib; their basilar arteries remained normal sized and strong. “Establishing the first non-surgical animal model of intracranial aneurysm is in itself an achievement,” says Nakatomi, “but more importantly, we suppressed artery expansion with a drug, indicating that intracranial aneurysms can be pharmacologically treated.”
Additional research will be required to demonstrate that this kind of drug treatment is effective for human patients. But perhaps the more difficult hurdle will be detection. As Nakatomi explains, “unruptured intracranial aneurysms are usually detected by Magnetic Resonance Angiography or Computed Tomography Angiography during health checkups. If these tests are not available, then aneurysms are undetectable until they burst.” In Japan, where this research was conducted, many people can receive these tests as part of their annual health checkup, making the development of drug treatments particularly useful.
—
Reference: Shima Y, et al. (2023) Increased PDGFRB and NF-κB signaling caused by highly prevalent somatic mutations in intracranial aneurysms. Sci Transl Med. DOI: 10.1126/scitranslmed.abq7721
Journal
Science Translational Medicine
DOI
10.1126/scitranslmed.abq7721
Article Title
Increased PDGFRB and NF-κB signaling caused by highly prevalent somatic mutations in intracranial aneurysms




