Credit: IDIBELL
Researchers of the Sarcoma research group of the Bellvitge Biomedical Research Institute (IDIBELL), led by Dr. Òscar Martínez-Tirado, have first described the methylation profile of Ewing's sarcoma (ES), a cancer of bone and soft tissues that mainly affects children and teenagers. Their analysis has unveiled the potential of the PTRF gene as a prognostic marker of the disease and as a possible future therapeutic target in conjunction with the new genomic editing tools available.
"This is the first time that the methylation profile of Ewing's sarcoma has been described," explains Dr. Òscar Martínez-Tirado, lead author of the study. DNA methylation is a type of epigenetic modification capable of controlling gene expression, that is, it can activate or deactivate genes that enhance to or block characteristics and processes in individuals, including the development of tumors. Using bioinformatic tools, the research team looked for common elements that allowed them to relate the set of differentially hypomethylated or hypermethylated genes they found in the tumor samples.
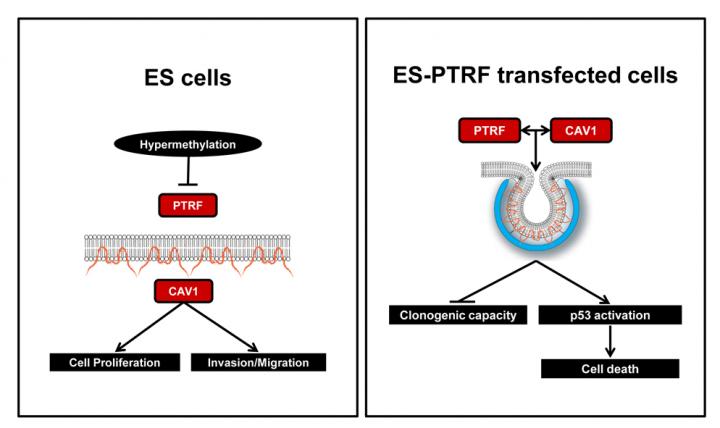
The researchers identified a group of 12 genes related to the structure of the cell membrane; Among them, the precursor of the protein PTRF particularly called their attention by its relation with the caveolae, small invaginations that are present in the membrane of some cells; the IDIBELL group has extensive experience in the study of these structures and their relationship with ES.
The team correlated the presence of PTRF in samples from 67 patients. "Initially, we saw that those patients who express PTRF have a better survival, therefore, we could consider this protein as a potential marker of prognosis of the disease," concludes Martinez-Tirado.
Normal cells with caveolae express both the PTRF protein and caveolin-1 (CAV1), another protein related to caveolae formation. In contrast, tumor cells in ES do not have caveolae, nor express PTRF. "This made us think about the possibility of exogenously reintroducing the precursor gene PTRF into the study cell lines", explains the researcher. "In those that also expressed CAV1, the reintroduction of PTRF triggered caveolae formation. For tumor cells, this modification of the structure is so stressful that it destroys them". In addition, researchers have shown that the formation of the caveolae in these cells activates a known cell death signaling pathway promoted by the p53 protein.
Current epigenetic drugs are rather nonspecific, but new CRISPR genomic editing tools have the potential to be used to demethylate specific genes from a tumor cell in the future. "If we are able to specifically act on the PTRF promoter gene so that the protein is expressed at normal levels, we would induce cell death and therefore we would have a promising new personalized therapeutic option for ES."
This has been a highly collaborative work, both at the research level, as researchers by two groups from IDIBELL's epigenetics and cancer biology program, Germans Trias Institute, Institute of biomedicine of Seville, University Hospital la Paz, Children's University Hospital Niño Jesús, San Juan de Dios Hospital, Vall d'Hebron University Hospital and Virgen del Rocío University Hospital have collaborated, as well as at a financial level level, given that the project is funded by the Carlos III Health Institute, the AECC and the Alba Pérez Foundation.
###
Media Contact
Gemma Fornons
[email protected]
0034-638-685-074
@idibell_en
############
Story Source: Materials provided by Scienmag