In order to keep track of their environment, cells use cilia, antenna-like structures that can sense a variety of stimuli, including the flow of fluids outside the cell. Genetic defects that cause cilia to malfunction and lose their sensory abilities can result in disorders known as “ciliopathies”, including polycystic kidney diseases; but they can also disrupt the correct asymmetric positioning of internal organs during embryonic development – what is known as “organ laterality”.
Credit: Benjamin Rothé and Zhidian Zhang (EPFL)
In order to keep track of their environment, cells use cilia, antenna-like structures that can sense a variety of stimuli, including the flow of fluids outside the cell. Genetic defects that cause cilia to malfunction and lose their sensory abilities can result in disorders known as “ciliopathies”, including polycystic kidney diseases; but they can also disrupt the correct asymmetric positioning of internal organs during embryonic development – what is known as “organ laterality”.
An example of such asymmetry is the heart, which is typically located on the left side, and positioning its blood vessels correctly in a left-right asymmetrical arrangement is critical for efficient oxygen supply around the body. “Therefore, insights into the molecular mechanisms that mediate the sensory functions of cilia to regulate organ laterality are important,” says Professor Daniel Constam at EPFL’s School of Life Sciences (Swiss Institute for Experimental Cancer Research).
In a new study, researchers led by Constam and Professor Matteo Dal Peraro (EPFL Institute of Bioengineering), have found that the factor which is activated by flow-sensing cilia to specify organ laterality is tightly regulated by two other ciliopathy-associated proteins whose molecular functions have been elusive until now. The study is published in PLoS Biology.
It takes three to tango
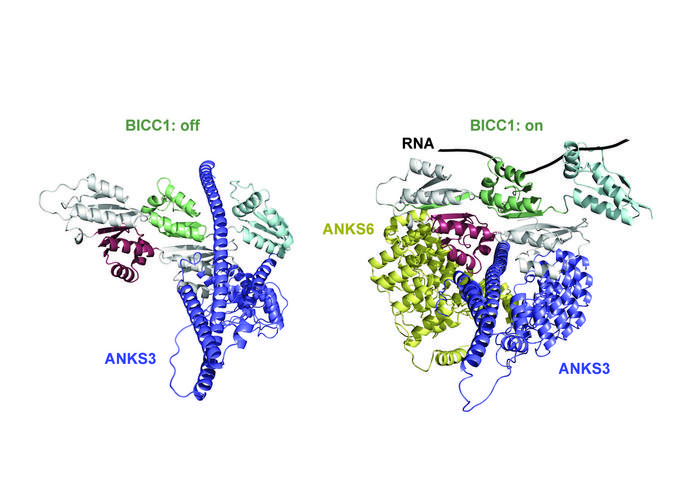
We already knew that flow stimulation of cilia determines left-right asymmetry by activating a protein known as Bicaudal-C1 (BICC1). BICC1 binds specific messenger RNAs (mRNAs) inside the cell to accelerate their decay, but specifically on the future left side of the body – like a switch that regulates what, where, and how much of a tissue will be “manufactured” in that particular location. What we didn’t know was how this mRNA-binding activity is itself regulated.
In the new study, the researchers discovered that mRNA binding of BICC1 is jointly regulated by two other proteins, ANKS3 and ANKS6, in an intricate protein network: “We focused on ANKS3 and ANKS6 because both proteins were recently implicated in regulating organ laterality,” says Constam. “ANKS6 is also mutated in a subset of nephronophthisis patients. However, how ANKS3 and ANKS6 function at the molecular level remained to be identified.”
The scientists found that the network of ANKS3 and ANKS6 with BICC1 engages multiple contact sites in an elegant molecular dance: ANKS3 competes with mRNAs for binding BICC1, but is in turn modulated by the protein ANKS6 to control how it interacts with BICC1. These structural changes in the ANKS3-Bicc1 complex, induced by ANKS6, determine whether BICC1 can access specific mRNAs or not.
Fundamental biology and potential treatments for genetic disorders
“Multivalent protein-RNA interaction networks are typically governed by disordered regions in proteins,” says Constam. “By contrast, we found that the BICC1 network is mediated by specific surfaces of well-structured protein domains that either compete or cooperate with one another. The cooperation between ANKS3 and ANKS6 to license BICC1 binding to specific mRNAs represents a new paradigm in the regulation of gene expression.”
Besides contributing to our fundamental understanding of organ development, the study also opens important new research avenues into how this sophisticated switch can be leveraged by cilia and by future therapies of genetic disorders where flow-sensing by cilia is inhibited.
“It’s really the ciliopathies where our findings will matter,” says Daniel Constam. “Inborn organ laterality defects are not something that anybody is even trying to cure in our lifetime, whereas restoring sensory functions of cilia is a top priority for devastating chronic disorders such as polycystic kidney diseases and nephronophthisis.”
Other contributors
- RIKEN Center for Biosystems Dynamics
- EPFL Institute of Bioengineering
- National Center of Neurology and Psychiatry (NCNP), Japan
Reference
Benjamin Rothé, Yayoi Ikawa, Zhidian Zhang, Takanobu A. Katoh, Eriko Kajikawa, Katsura Minegishi, Sai Xiaorei, Simon Fortier, Matteo Dal Peraro, Hiroshi Hamada, Daniel B. Constam. Bicc1 ribonucleoprotein complexes specifying organ laterality are licensed by ANKS6-induced structural remodeling of associated ANKS3. PLoS Biology 21 September 2023. DOI: xxxxxxxx
Journal
PLoS Biology
DOI
10.1371/journal.pbio.3002302
Article Title
Bicc1 ribonucleoprotein complexes specifying organ laterality are licensed by ANKS6-induced structural remodeling of associated ANKS3
Article Publication Date
21-Sep-2023




