The cholera bacterium uses a grappling hook-like appendage to take up DNA, bind to nutritious surfaces and recognize ‘family’ members, EPFL scientists have found
Credit: David W. Adams, Blokesch lab – EPFL
Bacteria are everywhere. They are the most abundant form of life on our planet. Pick up just about any surface and its likely covered in bacteria. The aquatic environment is no different. Indeed, the ocean is full of small particles and debris, some inert, some highly nutritious. But how do bacteria differentiate between these surfaces, how do they hold onto them in moving water and how do they recognise each other so that they can work together?
Take the cholera bacterium, Vibrio cholerae, which infects the small intestine, causing diarrhoea and severe dehydration. It lives in salty water, such as seas, oceans, and estuaries, attaching itself to the shells of crustaceans. These exoskeletons are composed of a sugary polymer called chitin, and provide a rich source of food for the cholera bacterium – allowing it to grow and survive in the environment.
To do all this, V. cholerae uses an appendage that’s “a bit like a grappling hook” says lead researcher David Adams. “The idea is that bacteria can throw out these long ropes, hook onto something, and reel it back in”. These ‘ropes’ are actually the product of highly versatile nano-machines known as type IV pili, which are used by many different bacterial species for motility, sensing surfaces and sticking to them, and even taking up DNA from neighbouring bacteria. Consequently, type IV pili are considered critical for the environmental survival and pathogenesis of not just V. cholerae but a wide range of bacteria.
Over the course of the last decade or so, the group of Melanie Blokesch established that V. cholerae produces these ‘DNA-uptake’ pili only when growing on chitinous surfaces and showed that they are essential for DNA-uptake. But how exactly they functioned and what else they might be capable of doing had remained somewhat elusive and was therefore the focus of the current study published in Nature Microbiology.
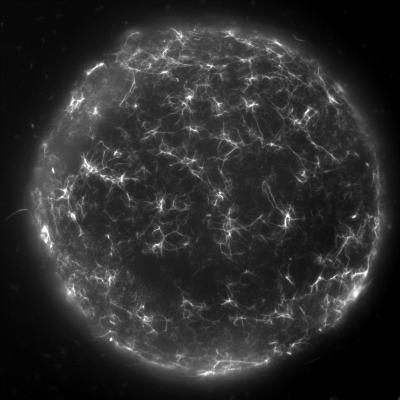
To directly observe the DNA-uptake pili in live V. cholerae bacteria, the researchers used a technique called cysteine labelling. With that, they were able to establish that, as predicted, the pili are highly dynamic, extending and retracting to take up DNA. “This was an important milestone” says laboratory head Melanie Blokesch “even though we’d established some time ago that these structures were there, to see them moving in real-time was something quite special”.
The biggest insight came however, when researchers disrupted the motor that powers pilus retraction, revealing that these ropes could also self-interact with each other, and in doing so, allow cells to stick together. Curiously, different strains of V. cholerae produce slightly different variants of the PilA subunit, which forms the major building block of the pilus. Remarkably, this creates a set of highly specific interactions that can be used as an identifier between strains ensuring that like only pairs with like.
Finally, when researchers visualised V. cholerae growing under more realistic conditions upon chitin surfaces, they revealed that these DNA-uptake pili naturally form dense networks of self-interacting pili. These pili bind tightly to the chitin surface and are required for the bacterium to stay attached during water flow. Thus, the DNA-uptake pilus is a multifunctional toolkit for chitin surface colonisation and kin recognition and the results of this work will help to advance our understanding of how the cholera bacterium survives in the natural environment. This knowledge, on the other hand, is important to better understand the transmission to humans in cholera endemic regions.
###
Media Contact
Melanie Blokesch
[email protected]
Related Journal Article
http://dx.




