Epigenetic regulator HPI1a drives de novo genome reorganization in early Drosophila embryos
Credit: MPI of Immunobiology and Epigenetics, F. Zenk
The DNA molecule is not naked in the nucleus. Instead, it is folded in a very organized way by the help of different proteins to establish a unique spatial organization of the genetic information. This 3D spatial genome organization is fundamental for the regulation of our genes and has to be established de novo by each individual during early embryogenesis. Researchers at the MPI of Immunobiology and Epigenetics in Freiburg in collaboration with colleagues from the Friedrich Mischer Institute in Basel now reveal a yet unknown and critical role of the protein HP1a in the 3D genome re-organization after fertilisation. The study published in the scientific journal Nature identifies HP1a as an epigenetic regulator that is involved in establishing the global structure of the genome in the early Drosophila embryo.
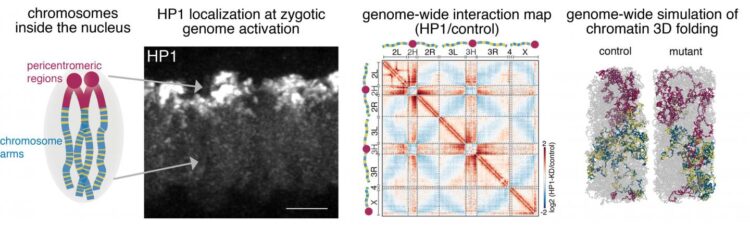
The information of the human genome is encoded by approximately 3 billion DNA base pairs and packaged into 23 pairs of chromosomes. If all chromosomes could be disentangled and linearly aligned, they would be a thin thread of about 2 meters. The DNA molecule must be extensively packaged to fit inside the nucleus, the size of which is in the micrometer range. “The DNA thread is not simply stuffed into the cell nucleus. Instead, it is folded in a very organized way to ensure that different parts of the genome, sometimes several thousand base pairs away from each other, can intercommunicate for appropriate gene functions,” says Nicola Iovino, group leader at the MPI of Immunobiology and Epigenetics in Freiburg.
Part of this packaging are histone proteins acting as spools around which DNA is winded and thereby compacted. This complex of DNA and proteins is called chromatin. As such, chromatin is the fundament for further packaging of the genetic material into chromosomes whose structure is mostly known for its characteristic cross shape. The chromosomes themself occupy distinct positions within the nucleus, known as chromosome territories, that also enable efficient packaging and organization of the genome.
The full machinery contributing to this 3D chromatin organization remains unexplored. Now the lab of Nicola Iovino at the MPI in Freiburg, in collaboration with Luca Giorgetti from the Friedrich Miescher Institute in Basel (Switzerland), was able to show the fundamental role of the heterochromatin protein 1a (HP1a) in the reorganization of the 3D chromatin structure after fertilization. By combining powerful Drosophila genetics with 3D genome modeling, they discovered that HP1a is required to establish a proper chromatin 3D structure at multiple hierarchical levels during early embryonic development.
Early embryos as a model to study chromatin reprogramming
The degree of packaging as well as the corresponding gene activity is influenced by epigenetic modifications. These are small chemical groups that are installed on the histones. “Proteins that carry out these epigenetic modifications can be thought of as being either writers, erasers or reader of the given epigenetic modification. We discovered that the reader protein HP1a is required to establish chromatin structure during early embryonic development in Drosophila”, says Fides Zenk, first-author of the study.
Early embryonic development is a particularly interesting time window to study the processes governing the organization of chromatin. At fertilization, two highly specialized cells – sperm and egg – fuse. The resulting totipotent zygote will ultimately give rise to all the different cells of the body. Interestingly most of the epigenetic modifications that shape chromatin are erased and have to be established de novo. In Drosophila, the lab of Nicola Iovino had previously shown that after fertilization chromatin undergoes major restructuring events. Thus, it is the ideal model system to study the processes underlying the establishment of chromatin structure.
De novo establishment of 3D genome architecture
When the genome of the zygote is activated for the first time after fertilization, it triggers global de novo 3D chromatin reorganization including a clustering of highly compacted regions around the centromere (pericentromeric), the folding of chromosome arms and the segregation of chromosomes into active and inactive compartments. “We identified HP1a as an important epigenetic regulator necessary to maintain individual chromosome integrity but also central for establishing the global structure of the genome in the early embryo,” says Nicola Iovino.
3D genome simulation
These findings and data collected in Drosophila embryos have then been used by collaborators from the Friedrich Miescher Institute (FMI) lead by Luca Giorgetti to build realistic three-dimensional models of chromosomes. This is possible because chromosomes inside the cell nucleus are polymers, very large molecules composed of chains of smaller components (monomers) – in this case consecutive DNA base pairs and the DNA-binding proteins that together constitute the chromatin fiber. Like all other polymers, be it silk, polyethylene or polyester, chromatin obeys a general set of physical laws described by a branch of physics known as ‘polymer physics’. These laws can be encoded into computer programs and used to simulate the three-dimensional shape of chromosomes in the nucleus.
“The advantage of this approach is that it allows simulating the effects of very large numbers of mutations. This enables researchers to explore scenarios that are beyond experimental reach, such as the simultaneous depletion of many different proteins that would require years of lab work. By comparing simulations with the outcome of experiments, this approach also allows to test alternative hypotheses concerning the mechanisms that lay at the basis of experimental observations,” says Luca Giorgetti, group leader at the Friedrich Miescher Institute in Basel.
In this case, FMI researchers used polymer models of the entire Drosophila genome to ask the question: based on the basic laws of polymer physics, is it possible that the depletion of a single protein – HP1 – leads to a massive change in the associations and shape of chromosomes in the nucleus? Or are additional mechanisms needed to explain the experimental observations? “We found that removal of the protein to its binding sites in the simulations accounted for the full set of experimental results, thus providing further confirmation that HP1 plays a key role in establishing the three-dimensional structure of the genome” says Yinxiu Zhan, co-first-author of the study.
###
Original publication
Zenk F, Zhan Y, Kos P, Löser E, Atinbayeva N, Schächtle M, Tiana G, Giorgetti L, Iovino N
HP1 drives de novo 3D genome reorganization in early Drosophila embryos
Nature (April 14, 2021)
Media Contact
Dr. Nicola Iovino
[email protected]
Original Source
https:/
Related Journal Article
http://dx.