Tumors have significant differences depending on the person affected, even if they are the same cancer, such as breast cancer. Therefore, precision oncology targets specific genetic characteristics of a tumor and incorporates them into treatment. In this way, existing therapies can be “tailored” to avoid side effects and save money on expensive treatments. This represents the cancer treatment of the future.
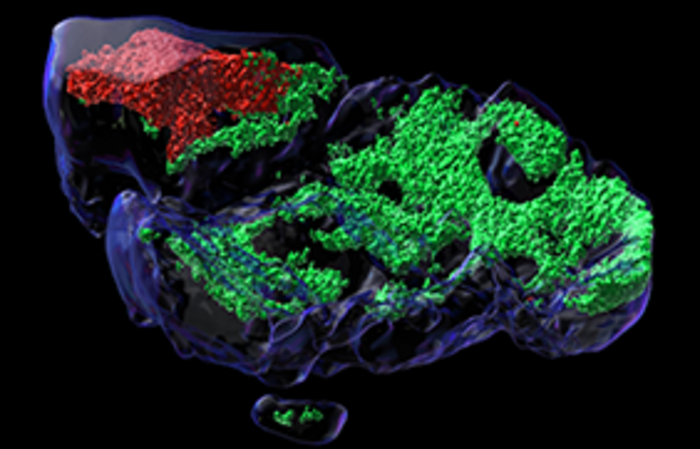
Credit: © Alison Ferguson / BCPM
Tumors have significant differences depending on the person affected, even if they are the same cancer, such as breast cancer. Therefore, precision oncology targets specific genetic characteristics of a tumor and incorporates them into treatment. In this way, existing therapies can be “tailored” to avoid side effects and save money on expensive treatments. This represents the cancer treatment of the future.
Dr Dilara Akhoundova, medical oncologist at the University Hospital Bern and postdoctoral researcher at the University of Bern, and Prof Mark A. Rubin, director of the Department for BioMedical Research (DBMR) and the Bern Center for Precision Medicine (BCPM), have now summarized and reviewed the most recent advances in multi-omics tumor profiling. In their review, published in the leading journal Cancer Cell, they provide a critical view of the current state of translational validation of the reviewed technologies and analyze their potential for integration into precision treatment. “These new technologies take us to a depth of understanding of tumors that has never been seen before. It is as if with the standard tools, we were told that Switzerland is a country with higher altitudes than the Netherlands; with these new technologies, we can see the 3-D landscape of mountains, valleys, and lakes,” says Mark A. Rubin, Director of the Bern Center for Precision Medicine.
Integrating novel technologies into the clinic as fast as possible
However, there are still a number of hurdles to overcome before the latest technologies can be used in the clinic: among other things, they still need to be standardized, or require new infrastructures in clinics due to the evaluation of a very large volume of data or regulatory approval.
One of the latest promising technologies in precision oncology is liquid biopsy, which makes it possible to provide information about the type of cancer in patients more quickly and minimally invasively by means of a blood test. Especially in the case of tumors located deep in the body, such as in the lungs or pancreas, this requires invasive procedures, occasionally under general anesthesia. Many such technologies as liquid biopsy are being used in translational and clinical cancer research. Their clinical potential is already very high; they still require, in some cases, an additional method that increases “measurement accuracy” for certain samples. Other innovations are still in their infancy and need to be clinically validated to see if they can even achieve their goal.
Bern initiatives for cancer research in Switzerland
Advancing and implementing novel, cutting-edge technologies into precision medicine is a crucial focus of BCPM. Cancer translational research projects led by researchers at the BCPM, such as those led by Prof. Mark A. Rubin, Prof. Marianna Kruithof-De Julio, and Prof. Sven Rottenberg, as well as a tight collaboration with clinical oncologists from the University Hospital Bern and other Swiss institutions, are essential to advance precision oncology further, and approach novel technologies to the patients. “In our review, we consider how these new approaches can be translated into tests that can better predict responses to tumor therapies in patients,” says Dilara Akhoundova, lead author of the study.
Another important Bern precision oncology initiative is the Swiss Oncology and Cancer Immunology Breakthrough Platform (SOCIBP), which aims to establish a common genomic “language” for Swiss cancer research: Molecular tumor data will be presented and shared in an understandable way, and genomic testing across Switzerland will be standardized. The project is funded by the Swiss Personalized Health Network (SPHN), a federal initiative. “One of our current translational projects focuses on the standardization and clinical validation of genomic tests assessing DNA repair in prostate cancer and other solid tumors”, Rubin explains. The project’s overarching goal is to develop more reliable predictive biomarkers allowing precision oncology treatment for tumors harboring DNA repair defects.
The study was supported by the Swiss Personalized Health Network (SPHN) SOCIBP, the Swiss Cancer League, the Nuovo-Soldati Foundation for Cancer Research, the ISREC Fondation Recherche Cancer, and the Werner and Hedy Berger-Janser Foundation.
DOI
10.1016/j.ccell.2022.08.011




