Although a plethora of carbon-based catalysts have been developed to promote oxygen reduction reaction (ORR) in different electrochemical systems, the degradation process of those catalysts remains obscure to date. During certain steps of the ORR on a catalyst’s surface in electrochemical systems, hydrogen peroxide (H2O2) is generated. This compound can be detrimental to the catalyst itself because the highly oxidative species produced from H2O2 can attack different moieties of the catalyst, leading to the degradation of its chemical structure. A team of researchers has elucidated how H2O2 affects the degradation of a carbon-based catalyst named N-G/MOF. This catalyst consists of N-doped graphene integrated with a metal-organic framework; a synthesis carried out by the same research team. They also examined the consequences of this degradation on the catalyst’s performance. Their work is published in the journal Industrial Chemistry & Materials on AUGUST 1, 2023.Although a plethora of carbon-based catalysts have been developed to promote oxygen reduction reaction (ORR) in different electrochemical systems, the degradation process of those catalysts remains obscure to date. During certain steps of the ORR on a catalyst’s surface in electrochemical systems, hydrogen peroxide (H2O2) is generated. This compound can be detrimental to the catalyst itself because the highly oxidative species produced from H2O2 can attack different moieties of the catalyst, leading to the degradation of its chemical structure. A team of researchers has elucidated how H2O2 affects the degradation of a carbon-based catalyst named N-G/MOF. This catalyst consists of N-doped graphene integrated with a metal-organic framework; a synthesis carried out by the same research team. They also examined the consequences of this degradation on the catalyst’s performance. Their work is published in the journal Industrial Chemistry & Materials on August 1, 2023.
Credit: Eon Soo Lee, Advanced Energy Systems and Microdevices Laboratory, Department of Mechanical and Industrial Engineering, New Jersey Institute of Technology, Newark, NJ, USA
Although a plethora of carbon-based catalysts have been developed to promote oxygen reduction reaction (ORR) in different electrochemical systems, the degradation process of those catalysts remains obscure to date. During certain steps of the ORR on a catalyst’s surface in electrochemical systems, hydrogen peroxide (H2O2) is generated. This compound can be detrimental to the catalyst itself because the highly oxidative species produced from H2O2 can attack different moieties of the catalyst, leading to the degradation of its chemical structure. A team of researchers has elucidated how H2O2 affects the degradation of a carbon-based catalyst named N-G/MOF. This catalyst consists of N-doped graphene integrated with a metal-organic framework; a synthesis carried out by the same research team. They also examined the consequences of this degradation on the catalyst’s performance. Their work is published in the journal Industrial Chemistry & Materials on AUGUST 1, 2023.Although a plethora of carbon-based catalysts have been developed to promote oxygen reduction reaction (ORR) in different electrochemical systems, the degradation process of those catalysts remains obscure to date. During certain steps of the ORR on a catalyst’s surface in electrochemical systems, hydrogen peroxide (H2O2) is generated. This compound can be detrimental to the catalyst itself because the highly oxidative species produced from H2O2 can attack different moieties of the catalyst, leading to the degradation of its chemical structure. A team of researchers has elucidated how H2O2 affects the degradation of a carbon-based catalyst named N-G/MOF. This catalyst consists of N-doped graphene integrated with a metal-organic framework; a synthesis carried out by the same research team. They also examined the consequences of this degradation on the catalyst’s performance. Their work is published in the journal Industrial Chemistry & Materials on August 1, 2023.
“We aim to elucidate the comprehensive mechanism of the electrocatalytic performance reduction and the concurrent degradation process of materials in carbon-based catalysts during their operation in electrochemical energy systems,” explains Eon Soo Lee, an associate professor at the New Jersey Institute of Technology, who leads the research team. Hydrogen peroxide (H2O2) unavoidably forms as a byproduct during the ORR process in various electrochemical energy systems. In the electrochemical reaction environments, this compound is unstable and readily breaks down into various highly oxidative species, which can profoundly impact the chemical structure of the catalysts, including carbon-based ones. While previous investigations have explored the effects of H2O2 on transition metal-based catalysts, this study represents the first in-depth analysis of the H2O2-driven material degradation process and its corresponding decline in electrocatalytic performance specifically in a carbon-based catalyst for ORR.
The importance of advancing electrochemical energy technologies to meet the world’s energy needs in the coming decades cannot be overstated. Currently, many electrochemical energy systems utilize expensive Platinum-group metal (PGM)-based catalysts, rendering these systems economically uncompetitive compared to combustion-based energy systems. In this context, various carbon-based functional materials have displayed potential, but there is still much work to be done before we can confidently employ carbon-based catalysts in electrochemical energy systems.
In particular, it is essential to have a comprehensive grasp of how catalyst materials degrade during their operation. This aspect is directly linked to the long-term performance and mechanical stability of electrochemical systems. Despite significant efforts to enhance the electrocatalytic performance of carbon-based catalysts, striving to bring them closer to the performance levels of Platinum-group metal (PGM)-based ones, the issue of degradation remains relatively uncharted territory. This is primarily due to the intricate structural and functional characteristics of carbon-based catalysts.
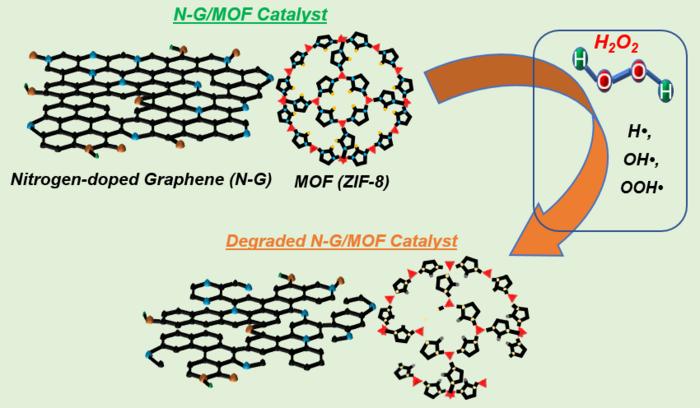
One prominent catalyst degradation pathway is linked to H2O2. It’s known that carbon-based catalysts, in particular, generate H2O2 as a byproduct during the oxygen reduction reaction (ORR). The key question is how H2O2 affects the catalyst’s chemical structure and, consequently, its performance stability. Understanding which chemical moieties are most affected by H2O2 and how these structural changes relate to alterations in electrocatalytic performance is crucial for carbon-based catalysts.
The research team used the N-G/MOF carbon-based catalyst and exposed it to varying concentrations of hydrogen peroxide (H2O2), mimicking real-world electrochemical energy system conditions. They examined changes in the catalyst’s elemental composition, major chemical bonds, crystal structure, and morphology. They also assessed the catalyst’s electrocatalytic performance. The study found that H2O2 primarily affected carbon-carbon bonds at higher concentrations, reduced the pyridinic-N component, which in turn impacted catalytic performance, and caused structural changes unfavorable for ORR catalysis.
Looking forward, the research team expects that their study could offer valuable insights into how H2O2-induced degradation affects various carbon and metal-organic framework-based electrocatalysts used in the oxygen reduction reaction (ORR) across diverse electrochemical systems. “In our next steps, we intend to perform an in-situ analysis of material degradation and the subsequent loss in electrocatalytic performance for the N-G/MOF catalysts. These studies, we hope, will establish a knowledge base for future efforts to examine the degradation process of carbon-based catalysts from various application-oriented viewpoints,” said Lee.
The research team includes Niladri Talukder, Yudong Wang, and Eon Soo Lee from the New Jersey Institute of Technology, Newark, New Jersey; Bharath Babu Nunna from Weber State University, Ogden, Utah; Xiao Tong and Jorge Anibal Boscoboinik from the Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York.
This research was conducted at the Advanced Energy System and Microdevices (AESM) Laboratory at the New Jersey Institute of Technology. Different material characterization facilities were used at the Center for Functional Nanomaterials (CFN), which is a U.S. Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. Also, some of the experiments were conducted at the Materials Characterization Laboratory at Otto H. York Center for Environmental Engineering and Science (YCEES) at New Jersey Institute of Technology.
Industrial Chemistry & Materials is a peer-reviewed interdisciplinary academic journal published by Royal Society of Chemistry (RSC) with APCs currently waived. Icm publishes significant innovative research and major technological breakthroughs in all aspects of industrial chemistry and materials, especially the important innovation of the low-carbon chemical industry, energy, and functional materials.
Journal
Industrial Chemistry and Materials
DOI
10.1039/D3IM00044C
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Investigation on electrocatalytic performance and material degradation of an N-doped graphene-MOF nanocatalyst in emulated electrochemical environments
Article Publication Date
1-Aug-2023




