When pathogens attack the body, the innate immune system goes to work protecting against the invading disease. The innate immune system is the first line of defense. It detects precisely what the virus or bacteria is and then activates the proteins that fight the pathogens. Wanting to better understand how the body’s innate immune system works, a team of scientists undertook a study of STING, a protein that plays a vital role in innate immunity.
Credit: Institute for Glyco-core Research
When pathogens attack the body, the innate immune system goes to work protecting against the invading disease. The innate immune system is the first line of defense. It detects precisely what the virus or bacteria is and then activates the proteins that fight the pathogens. Wanting to better understand how the body’s innate immune system works, a team of scientists undertook a study of STING, a protein that plays a vital role in innate immunity.
The team provides quantitative results, showing how STING, an acronym for stimulator of interferon genes, works in innate immune signaling.
Their work is published in the journal Nature Communications on Jan 11th, 2024.
Type I interferons are signaling proteins that respond when they detect the presence of viruses. They play an essential role in the body’s immune system, communicating between cells as they fight against pathogens. STING is critical for the type I interferon response to pathogen- or self-derived DNA in the cytosol, the fluid portion of a cell. While STING plays an important role in the body’s successful protection against infections, dysregulated STING activity leads to the excessive production of inflammatory mediators that can have a detrimental effect on surrounding cells and tissues. Recent studies have made the connection between STING and a number of autoinflammatory and neurodegenerative diseases.
“STING was discovered as a protein that induces innate immune signals in response to virus-derived non-self DNA. The STING innate immune response has recently been reported to play an important role in cancer immune responses and to contribute to inflammatory pathologies in aging, autoinflammatory, and neurodegenerative diseases, making it a highly attractive target for disease therapy,” said Kenichi G.N. Suzuki, a professor at the Institute for Glyco-core Research, Gifu University and a chief at the Division of Advanced Bioimaging, National Cancer Center Research Institute.
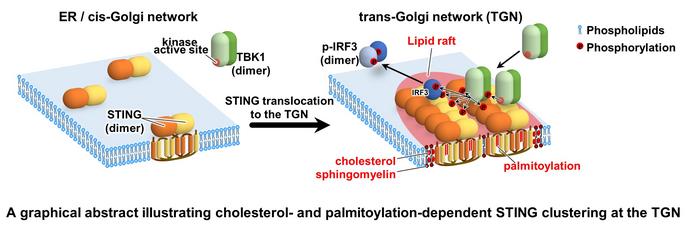
Research suggests that STING may function as a scaffold to activate the TANK-binding kinase 1 (TBK1). TBK1 is a signaling molecule that is activated by receptors when a viral infection occurs. Scaffold proteins do the important job of regulating key signaling pathways. However, up to this point, scientists have lacked direct cellular evidence proving that STING activated the TBK1.
To analyze the STING cluster, the research team used a live-cell imaging procedure called photoactivated localization microscopy or PALM. They performed this single-molecule imaging of STING with enhanced time resolutions down to 5 milliseconds. They determined that STING becomes clustered at the trans-Golgi network. The trans-Golgi network, or TGN, is a pathway in the body that directs proteins to the correct subcellular destination.
The team also proved that STING palmitoylation facilitated the STING clustering. Palmitoylation describes a protein modification process in the body. This palmitoylation of STING is required for the cluster formation of STING at the TGN. The Golgi lipid order, along with STING palmitoylation, is essential for the STING signaling. The team examined the role of cholesterol, a lipid that plays an essential role in generating the lipid order in STING’s signaling and clustering.
They used a cholesterol biosensor and an environmentally sensitive probe for lipid membranes, to further demonstrate that cholesterol plays a role in the palmitoylated STING-formed clusters that activate TBK1 at the TGN.
The team specifically examined the formation of STING clusters as it relates to COPA syndrome. COPA syndrome is a disorder of immune dysregulation characterized by an increase in type I interferon-stimulated genes. This autoimmune disorder can impact multiple systems in the body.
The team’s imaging of TBK1 revealed that the increase in the clustering enhances the association of TBK1. “We provide quantitative proof-of-principle for the signaling STING scaffold, reveal the mechanistic role of STING palmitoylation in the STING activation, and resolve the long-standing question of the requirement of STING translocation for triggering the innate immune signaling,” said Tomohiko Taguchi, a professor in the Graduate School of Life Sciences, Tohoku University.
Looking ahead, the team sees potential for this work helping the fight against disease. “In the present study, we showed that inhibition of cholesterol transport to TGN markedly suppressed the STING innate immune response. Therefore, based on the results of this study, it is expected that reducing cholesterol levels will be a new tool to treat the diseases associated with STING inflammation,” said Suzuki.
Haruka Kemmoku, Kanoko Takahashi, Kojiro Mukai, Yasunori Uchida, Yoshihiko Kuchitsu, and Tomohiko Taguchi from Tohoku University; Toshiki Mori, Koichiro M. Hirosawa, and Yasunari Yokota from Gifu University; Fumika Kiku and Hiroyuki Arai from the University of Tokyo; Yu Nishioka and Masaaki Sawa from Carna Biosciences, Inc.; Takuma Kishimoto and Kazuma Tanaka from Hokkaido University; and Kenichi G.N. Suzuki from Gifu University and the National Cancer Center, Tokyo.
This work was funded by JSPS KAKENHI, JSPS Research Fellowship for Young Scientists, AMED-PRIME, JST CREST, Subsidy for Interdisciplinary Study and Research concerning COVID-19 (Mitsubishi Foundation), National Cancer Center Research and Development Fund, Takeda Science Foundation, The Uehara Memorial Foundation, Mizutani Foundation for Glycoscience, Daiichi Sankyo Foundation of Life Science, Research Foundation for Opto-Science and Technology, The Naito Foundation, Grant for Basic Science Research Projects from the Sumitomo Foundation, SGH Cancer Research Grant, Research Grant of the Princess Takamatsu Cancer Research Fund, and the Nagoya University CIBoG program from MEXT WISE program.
###
About iGCORE:
Institute for Glyco-core Research (iGCORE) is a cutting-edge integrative glycoscience institute that brings together researchers from two universities – Nagoya University and Gifu University under the Tokai National Higher Education and Research System – with outstanding achievements in the fields of glycan synthesis, imaging, glycobiology, and glycomedicine. Through our research, iGCORE is committed to gaining a deeper understanding of the fundamental nature of life, ultimately paving the way for groundbreaking innovations in medicine, such as personalized prevention and early detection of pre-disease.
About The National Cancer Center Research Institute:
The National Cancer Center Research Institute is one of the largest cancer research institutions in Japan, with over 350 staff, including postgraduate students and research assistants. The Institute covers 20 research areas with 9 independent units, as well as the Fundamental Innovative Oncology Core, established as a common platform serving the entire Center. From highly original basic research to the development of therapeutic and diagnostic drugs, the Institute conducts a wide range of activities in collaboration with other units within the Center.
Journal
Nature Communications
DOI
10.1038/s41467-023-44317-5
Method of Research
Experimental study
Article Title
Single-molecule localization microscopy reveals STING clustering at the trans-Golgi network through palmitoylation-dependent accumulation of cholesterol
Article Publication Date
11-Jan-2024




