Ikoma, Japan – Pluripotent stem cells (PSCs) are a type of stem cells capable of developing into various cell types. Over the past few decades, scientists have been working towards the development of therapies using PSCs. Thanks to their unique ability to self-renew and differentiate (mature) into virtually any given type of tissue, PSCs could be used to repair organs that have been irreversibly damaged by age, trauma, or disease.
Credit: Atsushi Intoh
Ikoma, Japan – Pluripotent stem cells (PSCs) are a type of stem cells capable of developing into various cell types. Over the past few decades, scientists have been working towards the development of therapies using PSCs. Thanks to their unique ability to self-renew and differentiate (mature) into virtually any given type of tissue, PSCs could be used to repair organs that have been irreversibly damaged by age, trauma, or disease.
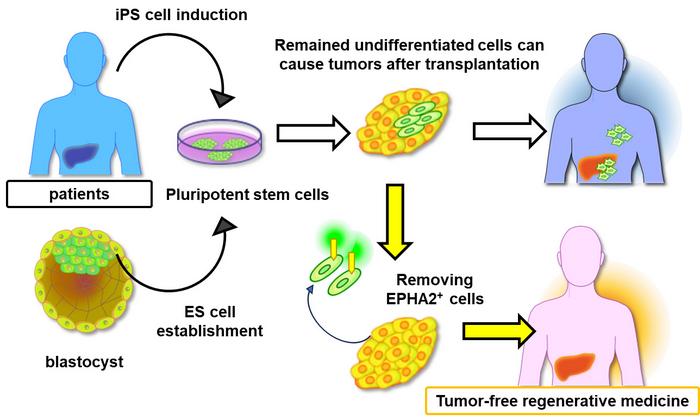
However, despite extensive efforts, regenerative therapies involving PSCs still have many hurdles to overcome. One being the formation of tumors (via the process of tumorigenesis) after the transplantation of PSCs. Once the PSCs differentiate into a specific type for stem cell therapy, there is a high probability of tumor formation after differentiated stem cells are introduced to the target organ. For the success of PSC-based therapies, the need of the hour is to minimize the risk of tumorigenesis by identifying potentially problematic cells in cultures, prior to transplantation.
Against this backdrop, a research team led by Atsushi Intoh and Akira Kurisaki from Nara Institute of Science and Technology, Japan, has recently achieved a breakthrough discovery regarding stem cell therapy and tumorigenesis. “Our findings present advancements that could bridge the gap between stem cell research and clinical application,” says Intoh, talking about the potential of their findings. Their study was published in Stem Cells Translational Medicine and focuses on a membrane protein called EPHA2, which was previously found to be elevated in PSCs prior to differentiation by the team.
Through several experiments involving both mouse and human stem cell cultures, the researchers gained insights into the role of EPHA2 in preserving the potency of PSCs to develop into several cell types. They found that EPHA2 in stem cells is co-expressed with OCT4—a transcription factor protein which controls the expression of genes which are critically involved in the differentiation of embryonic stem cells. Interestingly, when the EPHA2 gene was knocked down from the cells, cultured stem cells spontaneously differentiated. These results suggest that EPHA2 plays a central role in keeping stem cells in an undifferentiated state.
The researchers thus theorized that EPHA2-expressing stem cells, which would fail to differentiate, might be responsible for tumorigenesis upon transplantation into the target organ.
To test this hypothesis, the researchers prepared PSC cultures and artificially induced their differentiation into liver cells. Using a magnetic antibody targeting EPHA2, they extracted EPHA2-positive cells from a group of cultures prior to transplantation into mice. Interestingly, the formation of tumors in mice receiving transplants from cultures from which EPHA2 had been removed was vastly suppressed.
Taken together, these results point to the importance of EPHA2 in emerging stem cell-based therapies. “EPHA2 conclusively emerges as a potential marker for selecting undifferentiated stem cells, providing a valuable method to decrease tumorigenesis risks after stem cell transplantation in regenerative treatments,” remarks Kurisaki.
Further in-depth studies on this protein may lead to the development of protocols that make PSCs safer to use. Luckily, however, these findings pave the way towards a future where we will be able to finally restore damaged organs and even overcome degenerative conditions.
###
Resource
Title: EPHA2 is a novel cell surface marker of OCT4-positive undifferentiated cells during the differentiation of mouse and human pluripotent stem cells.
Authors: Atsushi Intoh, Kanako Watanabe-Susaki, Taku Kato, Hibiki Kiritani, Akira Kurisaki
Journal: Stem Cells Translational Medicine
DOI: 10.1093/stcltm/szae036
Information about Laboratory for Stem Cell Technologies can be found at the following website: https://bsw3.naist.jp/eng/courses/courses215.html
About Nara Institute of Science and Technology (NAIST)
Established in 1991, Nara Institute of Science and Technology (NAIST) is a national university located in Kansai Science City, Japan. In 2018, NAIST underwent an organizational transformation to promote and continue interdisciplinary research in the fields of biological sciences, materials science, and information science. Known as one of the most prestigious research institutions in Japan, NAIST lays a strong emphasis on integrated research and collaborative co-creation with diverse stakeholders. NAIST envisions conducting cutting-edge research in frontier areas and training students to become tomorrow’s leaders in science and technology.
Journal
Stem Cells Translational Medicine
DOI
10.1093/stcltm/szae036
Method of Research
Experimental study
Subject of Research
Animals
Article Title
EPHA2 is a novel cell surface marker of OCT4-positive undifferentiated cells during the differentiation of mouse and human pluripotent stem cells.




