A paper by Kazan Federal University appeared in Amino Acids
Credit: Kazan Federal University
Coordination Compounds Lab of Kazan Federal University started researching prebiotic peptide synthesis in 2013 with the use of the ASIA-330 flow chemistry system. Many lab projects are devoted to the problem of selectivity and specificity of processes in living nature. This problem is directly related to prebiotic chemistry, whose foundations were laid as a result of the amino acids synthesis by the German chemist Adolph Friedrich Ludwig Strecker (the Strecker reaction, 1851) and of the sugar synthesis by the Russian chemist, the founder of the Kazan Chemical School, Alexander Butlerov (the formose reaction, 1861). Studies on the prebiotic synthesis of sugars and peptides are aimed at a deeper understanding of the most fundamental and intriguing problem of our time – the origin of life.
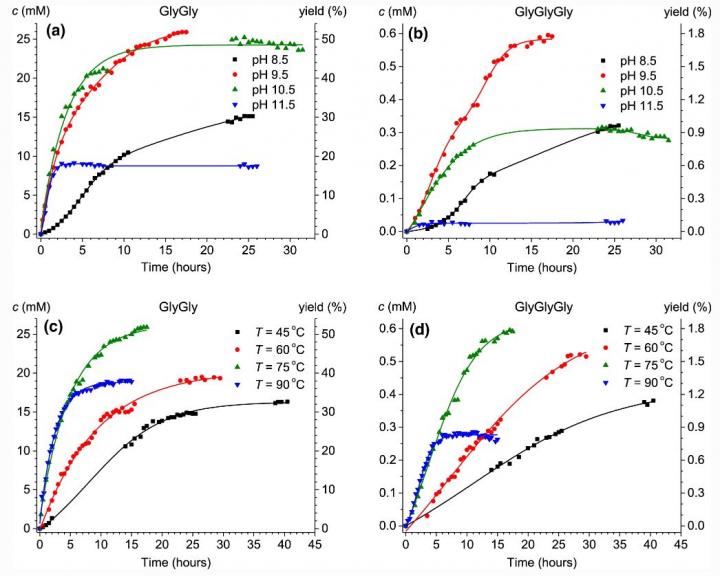
The kinetics of oligopeptides formation in the flow systems glycine – sodium trimetaphosphate – imidazole/N-methylimidazole at thermocyclic regime was investigated by liquid chromatography (HPLC) and 31P NMR methods in the ranges of temperature from 45 to 90°C and pH from 8.5 to 11.5. Formation of significant amounts of glycylglycine (yield up to 52%) and diglycylglycine was revealed. Better results are obtained at 75°C in slightly alkaline conditions (pH 9.5-10.5), and in the presence of imidazole yields of oligopeptides are bigger than without this heterocycle. It should be mentioned that the used non-equilibrium regime of the glycylglycine and diglycylglycine syntheses turns out to be one of the most effective among all prebiotic syntheses reported so far in the literature, both in the absence and in the presence of imidazole. Earlier, H. Sawai and L.E. Orgel discovered that heterocycles, such as imidazole, can increase yields of peptides in solid state (Sawai H., Orgel L.E. (1975) J. Mol. Evol. 6:185-197. doi:10.1007/bf01732355). However, the proposed explanation of the catalytic effect of imidazole due to initial formation of N-triphosphoryl imidazole as a key intermediate is not working in the reactions studied by us because in the imidazole – trimetaphosphate mixture signals of any imidazole N-phosphates are absent in 31P NMR spectra. In this situation, a new imidazole catalysis mechanism by which imidazole reacts with cyclic N,O-phosphoryl glycine giving N-imidazolyl-O-glycyl phosphate as a key intermediate was proposed and validated in the present investigation. Detailed reaction mechanisms were proposed and justified by quantum chemical calculations using density functional theory (DFT) method at the high level (CAM-B3LYP/TZVP) with accounting solvent effect by the polarized continuum model (?-PCM). It is emphasized that while in the absence of imidazoles, prebiotic activation of amino acids occurs at the N-terminus, and in the presence of imidazoles it shifts to the O-terminus. This means that in the peptide elongation N-imidazolyl-O-aminoacyl phosphates play in prebiotic systems the outstanding role similar to that of aminoacyl adenylates formed at the ATP and aminoacyl-tRNA synthetases presence in biosystems. This seems to be a key pathway for prebiotic evolution in terms of peptide synthesis. So, the new crucial role of imidazoles in prebiotic evolution was discovered. The systems used and modes of their conversion can be good models for prebiotic peptide syntheses in a flow thermocyclic regime, including prebiotic peptide syntheses under conditions of various hydrothermal systems, particulary in Kamchatka, where temperature and pressure fluctuations are detected and pH varies from 2.0 to 9.5 while temperature ranges from 55 to 98oC in hot springs.
The work is of importance for the development of the problem of prebiotic peptide synthesis. The results can be used in the synthesis of small oligopeptides. In addition, the used experimental setup in combination with mathematical modeling and quantum-chemical calculations can be used to study other processes in the thermocyclic mode. The fact that N-methylimidazole has catalytic effect on the prebiotic peptide synthesis is especially important because similar heterocycles can be formed under shock exposure at prebiotic conditions (Shtyrlin V.G. et al. (2019) Orig. Life Evol. Biosph. 49:1-18. doi:10.1007/s11084-019-09575-8). In the cited work, it was found that upon impact on the water – formamide – bicarbonate – sodium hydroxide system, placed in a stainless steel preservation capsule, 7 imidazole derivatives (out of 21 products) are formed. It was established that the most effective syntheses proceed at pH ~9.5, and ammonia and formaldehyde are formed among many intermediate products. Note that according to the results of the cited study, a new hypothesis about the origin of life was proposed: life could have originated due to the impact of meteorites on alkaline water-formamide lakes located near volcanoes on the early Earth.
Further research can pertain to the synthesis of oligopeptides containing other amino acids and the synthesis of other biopolymers, primarily sugars. Particular attention will be paid to experimental and theoretical studies of mechanisms of heterogeneous catalysis in prebiotic syntheses of biopolymers. The focus will be on the role of coordination and complexation with metals in prebiotic syntheses, since metal complexes could control the stereoselectivity and specificity of many vital processes at the first stages of biochemical evolution.
###
Media Contact
Yury Nurmeev
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




