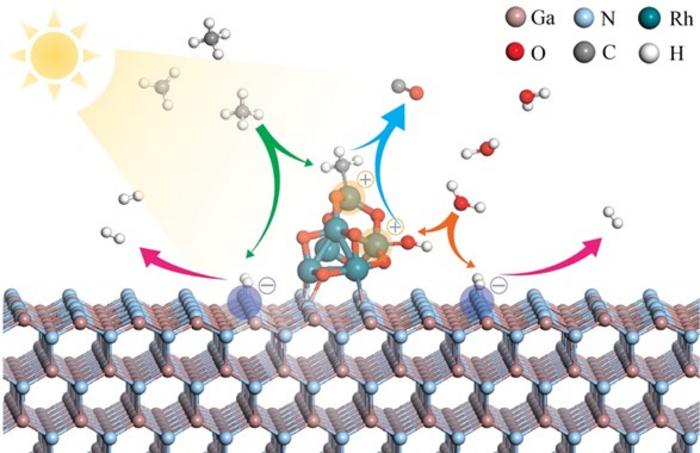
A study reports a photocatalyst to enable solar-driven syngas production from methane steam reforming—a possible bridge fuel to a post-carbon energy world. Methane steam reforming is the process of heating methane with steam in the presence of a catalyst to produce hydrogen and carbon monoxide, a mixture known as syngas, which can be used as fuel. The necessary reaction is difficult to achieve, however, and current industrial standard processes require high temperatures (700–1000 degrees C) and pressures (>20 bar). Baowen Zhou and colleagues present a photocatalysis platform that enables syngas production in a quartz chamber under atmospheric pressure illuminated by a 300 W Xenon lamp without any other energy inputs. The platform is based on group III nitride nanowires, fitted with rhodium nanoclusters. Analyses by theoretical calculations, microscopic characterizations, and in situ spectroscopic measurements show that these RhOx/GaN@InGaN nanowires simultaneously activate both methane and water. Just add light, and methane is split into methyl anions and hydrogen species. Water is split into hydrogen species and hydroxide. In subsequent reactions catalyzed by rhodium and gallium nitride, these molecules recombine into syngas. Using their system, the authors achieved a production rate of 8.1 mol syngas per gram of hydrogen and 10493 mol syngas per mol rhodium oxides over 300 minutes of stability test.
Credit: Li et al.
A study reports a photocatalyst to enable solar-driven syngas production from methane steam reforming—a possible bridge fuel to a post-carbon energy world. Methane steam reforming is the process of heating methane with steam in the presence of a catalyst to produce hydrogen and carbon monoxide, a mixture known as syngas, which can be used as fuel. The necessary reaction is difficult to achieve, however, and current industrial standard processes require high temperatures (700–1000 degrees C) and pressures (>20 bar). Baowen Zhou and colleagues present a photocatalysis platform that enables syngas production in a quartz chamber under atmospheric pressure illuminated by a 300 W Xenon lamp without any other energy inputs. The platform is based on group III nitride nanowires, fitted with rhodium nanoclusters. Analyses by theoretical calculations, microscopic characterizations, and in situ spectroscopic measurements show that these RhOx/GaN@InGaN nanowires simultaneously activate both methane and water. Just add light, and methane is split into methyl anions and hydrogen species. Water is split into hydrogen species and hydroxide. In subsequent reactions catalyzed by rhodium and gallium nitride, these molecules recombine into syngas. Using their system, the authors achieved a production rate of 8.1 mol syngas per gram of hydrogen and 10493 mol syngas per mol rhodium oxides over 300 minutes of stability test.
Journal
PNAS Nexus
Article Title
A semiconducting hybrid of RhOx/GaN@InGaN for simultaneous activation of methane and water toward syngas by photocatalysis
Article Publication Date
21-Nov-2023